* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Macrolids
Discovery and development of antiandrogens wikipedia , lookup
Compounding wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Ciprofloxacin wikipedia , lookup
Drug design wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug discovery wikipedia , lookup
List of comic book drugs wikipedia , lookup
Dydrogesterone wikipedia , lookup
Theralizumab wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
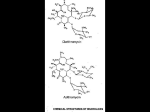
Macrolides Macrolides The macrolides are a group of closely related compounds characterized by a macrocyclic lactone ring (usually containing 14 or 16 atoms) to which deoxy sugars are attached. The prototype drug, erythromycin, which consists of two sugar moieties attached to a 14-atom lactone ring, was obtained in 1952 from Streptomyces erythreus. Clarithromycin and azithromycin are semisynthetic derivatives of erythromycin. Macrolid Antibiotics The antibacterial action of erythromycin may be inhibitory or bactericidal, depending on the drug concentration at the site of action. Activity is enhanced at alkaline pH. Inhibition of protein synthesis occurs via binding to the 50S ribosomal RNA, which blocks the aminoacyl translocation reaction and formation of initiation complexes. Macrolid Antibiotics Resistance Resistance to erythromycin is usually plasmid-encoded. Three mechanisms have been identified: (1) reduced permeability of the cell membrane or active efflux; (2) production (by Enterobacteriaceae) of esterases that hydrolyze macrolides; and (3) modification of the ribosomal binding site (so-called ribosomal protection) by chromosomal mutation or by a macrolide-inducible or constitutive methylase. Efflux and methylase production are by far the most important resistance mechanisms in gram-positive organisms. Cross-resistance is complete between erythromycin and the other macrolides. Macrolid Antibiotics Erythromycin (prototype) Antimicrobial spectrum Gram-positive organisms Pneumococci Streptococci Staphylococci Corynebacteria Mycoplasma Legionella Chlamydia trachomatis Chlamydia psittachi Helicobacter Listeria Gram-negative organisms Rickettsia Neisseria species Bordatella pertusis Bartonella henselae Bartonella quintana Treponema pallidum Campylobacter species Clinical Use Eerythromycin is the drug of choice in; corynebacterial infections (diphtheria, corynebacterial sepsis, erythrasma); in respiratory, neonatal, ocular, or genital chlamydial infections; and in treatment of community-acquired pneumonia because its spectrum of activity includes pneumococcus, mycoplasma, and legionella. Erythromycin is also useful as a penicillin substitute in penicillin-allergic individuals with infections caused by staphylococci (assuming that the isolate is susceptible), streptococci, or pneumococci. Emergence of erythromycin resistance in strains of group A streptococci and pneumococci (penicillin-resistant pneumococci in particular) has made macrolides less attractive as first-line agents for treatment of pharyngitis, skin and soft tissue infections, and pneumonia. Erythromycin has been recommended as prophylaxis against endocarditis during dental procedures in individuals with valvular heart disease, although clindamycin, which is better tolerated, has largely replaced it. Although erythromycin estolate is the best-absorbed salt, it imposes the greatest risk of adverse reactions. Therefore, the stearate or succinate salt may be preferred. Adverse Reactions A. GASTROINTESTINAL EFFECTS Anorexia, nausea, vomiting, and diarrhea occasionally accompany oral administration. Gastrointestinal intolerance, which is due to a direct stimulation of gut motility, is the most common reason for discontinuing erythromycin and substituting another antibiotic. B. LIVER TOXICITY Erythromycins, particularly the estolate, can produce acute cholestatic hepatitis (fever, jaundice, impaired liver function), probably as a hypersensitivity reaction. Most patients recover from this, but hepatitis recurs if the drug is readministered. Other allergic reactions include fever, eosinophilia, and rashes. C. DRUG INTERACTIONS Erythromycin metabolites can inhibit cytochrome P450 enzymes and thus increase the serum concentrations of numerous drugs, including theophylline, oral anticoagulants, cyclosporine, and methylprednisolone. Erythromycin increases serum concentrations of oral digoxin by increasing its bioavailability. Other Macrolides Clarithromycin Azithromycin Roxithromycin Clarithromycin Clarithromycin is derived from erythromycin by addition of a methyl group and has improved acid stability and oral absorption compared with erythromycin. Its mechanism of action is the same as that of erythromycin. Clarithromycin and erythromycin are virtually identical with respect to antibacterial activity except that clarithromycin is more active against Mycobacterium avium complex. Clarithromycin also has activity against M leprae and Toxoplasma gondii. Erythromycin-resistant streptococci and staphylococci are also resistant to clarithromycin. Clarithromycin The longer half-life of clarithromycin (6 hours) compared with erythromycin permits twice-daily dosing. The recommended dosage is 250-500 mg twice daily or 1000 mg of the extended release formulation once daily. Clarithromycin penetrates most tissues well, with concentrations equal to or exceeding serum concentrations. Clarithromycin is metabolized in the liver. The major metabolite is 14-hydroxyclarithromycin, which also has antibacterial activity. A portion of active drug and this major metabolite is eliminated in the urine, and dosage reduction (eg, a 500-mg loading dose, then 250 mg once or twice daily) is recommended for patients with creatinine clearances less than 30 mL/min. Clarithromycin has drug interactions similar to erythromycin (can inhibit cytochrome P450 enzymes). The advantages of clarithromycin compared with erythromycin are lower incidence of gastrointestinal intolerance and less frequent dosing. Except for the specific organisms noted above, the two drugs are otherwise therapeutically very similar, and the choice of one over the other usually turns out to be cost and tolerability. Azithromycin Azithromycin, a 15-atom lactone macrolide ring compound, is derived from erythromycin by addition of a methylated nitrogen into the lactone ring. Its spectrum of activity and clinical uses are virtually identical to those of clarithromycin. Azithromycin is active against M avium complex and T gondii. Azithromycin is slightly less active than erythromycin and clarithromycin against staphylococci and streptococci and slightly more active against H influenzae. Azithromycin is highly active against chlamydia. Azithromycin differs from erythromycin and clarithromycin mainly in pharmacokinetic properties. A 500-mg dose of azithromycin produces relatively low serum concentrations of approximately 0.4 mcg/mL. However, azithromycin penetrates into most tissues (except cerebrospinal fluid) and phagocytic cells extremely well, with tissue concentrations exceeding serum concentrations by 10- to 100-fold. The drug is slowly released from tissues (tissue half-life of 2-4 days) to produce an elimination half-life approaching 3 days. Azithromycin These unique properties permit once-daily dosing and shortening of the duration of treatment in many cases. For example, a single 1-g dose of azithromycin is as effective as a 7-day course of doxycycline for chlamydial cervicitis and urethritis. Community-acquired pneumonia can be treated with azithromycin given as a 500-mg loading dose, followed by a 250-mg single daily dose for the next 4 days. Azithromycin is rapidly absorbed and well tolerated orally. It should be administered 1 hour before or 2 hours after meals. Aluminum and magnesium antacids do not alter bioavailability but delay absorption and reduce peak serum concentrations. Because it has a 15-member (not 14-member) lactone ring, azithromycin does not inactivate cytochrome P450 enzymes and therefore is free of the drug interactions that occur with erythromycin and clarithromycin. Roxithromycin Similar to clarithromycin and erythromycin.