* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry The Chemistry Of Life / The Chemistry of Carbon
Survey
Document related concepts
Transcript
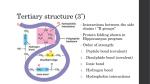
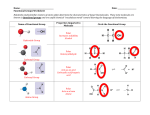
Organic Chemistry CD The Chemistry Of Life / The Chemistry of Carbon A. Historical Perspective 1. Vitalism: The belief that “life forces” outside of physical laws govern the interaction of chemicals in living things “Only living things can make organic compounds” 2. Mechanism: The belief that chemical interactions can be explained by physical laws “Any organic compound can be made in a test tube” Urea Properties of Carbon “Carbon is the tinker toy of life” Property of Carbon Biological Implication 1. Tetra valence 1. Carbon can form up to 4 covalent bonds for many “links” 2. Bond to itself 2. Carbon can form long chains or rings to form large molecules 3. Can form double and triple bonds 3. Different bond types will change the reactivity of the molecule 4. Weak yet strong bonds 4. Weak enough to metabolize and recycle but strong enough for stability 5. “Infinite” arrangements 5. Can form isomers Structural vs. Molecular formulas C4H10 a) structural b) geometric c) enantiomers CD Activity Functional Groups: Molecules that give Carbon Compounds Distinct Characteristics Personality: 1. Very Polar 2. OH groups on non-polar compounds allow the non-polar compounds to become soluble in water Personality: 1. Aldehyde : Polar, Found at the end of the molecule. Many are metabolic intermediates 2. Ketone: Non-Polar, Found in the Middle of the molecules Personality: 1. Polar 2. The “acid” part of an amino acid 3. Weak acid and can act as a buffer Personality: 1. Forms the Amine end of an amino acid 2. Polar 3. Tends to act like a base Personality: 1. Polar 2. Will form strong “di-sulfide bonds” (S-S) with other thiols 3. Will greatly influence shape of proteins Personality: 1. Very Polar 2. Important as: a) Energy transport (ATP), Destabilizes molecules b) Emulsifiers / Cell Membranes c) Structure of DNA CD Activity 1 CD Activity 2 Butane Isobutane Slide 1 The Tetra Valence of Carbon Slide 1 Carbon Can Bond to Itself Increasing Length Increasing Branching Allows Ring Structures Slide 1 Carbon can form Double and Triple Bonds Single Bonds Double Bonds Slide 1 Slide 1 Slide 1 Slide 1 CD Activity