* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Left Ventricular Diastolic Mechanical Dyssynchrony and Associated
Coronary artery disease wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
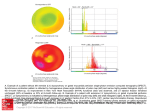
Left Ventricular Diastolic Mechanical Dyssynchrony and Associated Clinical Outcomes in Children With Dilated Cardiomyopathy Mark K. Friedberg, MD; Susan L. Roche, MD; Arshiya F. Mohammed; Mervin Balasingam; Eshetu G. Atenafu, MSc; Paul F. Kantor, MBBCh, DCH, FRCPC Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 Background—We investigated diastolic mechanical dyssynchrony and its relation to clinical status in pediatric dilated cardiomyopathy (DCM). Methods and Results—We calculated a diastolic and systolic dyssynchrony index (standard deviation of time to peak tissue early diastolic/systolic velocity in 12 left ventricular segments) in 33 children with DCM and 46 control subjects. A threshold to diagnose diastolic dyssynchrony was determined, and cardiac function and clinical outcomes were compared between DCM patients with and without diastolic dyssynchrony. Left ventricular wall motion was more synchronized in diastole than in systole. The diastolic dyssynchrony index was significantly higher in children with DCM than in control subjects (28.1⫾18.1 versus 9.1⫾3.8 ms, P⬍0.0001). A 17-ms threshold indicated the presence of diastolic dyssynchrony. Patients who died or underwent transplantation had greater diastolic dyssynchrony (diastolic dyssynchrony index 37.9⫾20.5 versus 22.1⫾13.8 ms, P⫽0.01), and the rate of transplant-free survival appeared to be worse for DCM patients with diastolic dyssynchrony than for patients with synchronous DCM (hazard ratio 2.98, P⫽0.11; hazard ratio adjusted for disease duration 2.95, P⫽0.17). Conclusions—Left ventricular diastolic mechanical dyssynchrony is common in pediatric DCM, especially in patients who subsequently experience transplantation or death, and may be associated with a decreased length of transplantation-free survival. (Circ Cardiovasc Imaging. 2008;1:50-57.) Key Words: pediatrics 䡲 cardiomyopathy 䡲 dyssynchrony, diastolic mechanical 䡲 echocardiography 䡲 imaging 䡲 survival M DCM. We hypothesized that diastolic wall-motion abnormalities are prevalent in pediatric DCM and that their presence is associated with diastolic ventricular dysfunction and adverse outcome. echanical dyssynchrony, the incoordinate wall motion of different ventricular segments in systole and diastole, is an important contributor to left ventricular (LV) dysfunction in cardiomyopathy both in adults and in children with dilated cardiomyopathy (DCM).1–3 Although systolic mechanical dyssynchrony has been studied more than diastolic mechanical dyssynchrony, diastolic dyssynchrony is more common than systolic dyssynchrony in adults with both systolic and diastolic heart failure and may be associated with ventricular dysfunction and poor outcome.4 –10 However, diastolic wall motion has not been well delineated in children in general, and diastolic mechanical dyssynchrony has not been studied in children with DCM. Therefore, the objectives of the present study were (1) to compare diastolic and systolic mechanical synchrony in normal control subjects and in patients with DCM; (2) to investigate the relation between diastolic dyssynchrony and cardiac function; and (3) to explore the relation between the presence of diastolic dyssynchrony and clinical status and outcome in patients with Clinical Perspective see p 57 Methods Population Children between 0 and 18 years of age who had undergone echocardiography between January 2006 and February 2008 were eligible for inclusion if they were being followed up for clinically significant DCM with decreased LV systolic function (LV ejection fraction ⬍55%). Clinical data were obtained retrospectively from the medical record. Healthy children investigated for an innocent murmur or for screening purposes were used as control subjects for evaluation of mechanical dyssynchrony. The study was approved by the institutional ethics board. Echocardiography Echocardiography was performed on a Vivid 7 echo system (GE Corp, Wauwatosa, Wis) with probe frequencies appropriate for Received March 24, 2008; accepted May 14, 2008. From the Divisions of Pediatric Cardiology, The Labatt Family Heart Center (M.K.F., S.L.R., A.F.M., M.B., P.F.K.) and Child Health Evaluative Sciences (E.G.A.), Hospital for Sick Children, Toronto, Ontario, Canada. Correspondence to Mark K. Friedberg, MD, Division of Cardiology, Hospital for Sick Children, 555 University Ave, Toronto, Ontario M5G 1X8, Canada. E-mail [email protected] © 2008 American Heart Association, Inc. Circ Cardiovasc Imaging is available at http://circimaging.ahajournals.org 50 DOI: 10.1161/CIRCIMAGING.108.782086 Friedberg et al Diastolic Dyssynchrony in Pediatric Cardiomyopathy patient size. Tissue Doppler imaging was acquired from equally rotated apical 4-, 3-, and 2-chamber views. The image position, depth, and sector width were optimized for frame rate and insonation angle. Frame rates obtained were 154⫾42 frames per second (mean⫾SD). Echocardiography data were transferred to an Echopac workstation (GE Corp) for offline analysis in which tissue Doppler sample volumes of 5 mm were placed at 12 LV segments (6 basal and 6 mid-LV segments), as well as at the tricuspid valve annulus. Echocardiographic analysis was blinded to clinical status and outcome; however, because of the obvious echocardiographic differences between DCM and normal conditions, it was not practical to blind the operator to diagnosis. 51 A Evaluation of Dyssynchrony LV Intraventricular Systolic Synchrony Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 We measured systolic synchrony by 2 methods. First, the LV intraventricular systolic delay was defined as the delay between time to peak systolic velocity (S⬘) at the mitral annulus and time to S⬘ at the basal septum, with the ECG QRS complex onset used as a reference.1 Next, the standard deviation (SD) of time to peak systolic velocity between 12 LV basal and mid-LV orthogonal segments was used as a dyssynchrony index (Figure 1).11,12 B LV Intraventricular Diastolic Synchrony Similarly, we measured LV intraventricular diastolic dyssynchrony by 2 methods: (1) the delay between time to peak early diastolic velocity (E⬘) at the mitral annulus and time to E⬘ at the basal septum, with the ECG QRS complex onset used as a reference, and (2) the SD of time to peak E⬘ of 12 basal and mid-LV orthogonal segments (Figure 1).9 In instances in which the E⬘ and A⬘ waves were blended, measurement was made to the peak of the single diastolic wave. The dyssynchrony index method constituted the primary method to determine the presence of systolic and diastolic mechanical dyssynchrony and to investigate the relation between dyssynchrony and clinical status. Left–Right Interventricular Systolic and Diastolic Synchrony Systolic interventricular delay was defined as the delay between time to peak S⬘ in the mitral annulus and time to peak S⬘ in the tricuspid annulus. Interventricular diastolic delay was defined as the delay between time to peak E⬘ in the mitral annulus and time to peak E⬘ in the tricuspid annulus. Statistical Analysis Data were analyzed with commercially available software (SAS version 9.1, SAS Institute Inc, Cary, NC). The normality assumption of continuous data was assessed by the Kolmogorov and Smirnov test. For data for which the normality assumption held, a comparison of 2 groups was assessed with the 2-tailed Student t test. The Welch correction was also applied when equality of variance could not be assumed between groups. A paired t test was used to compare the systolic and diastolic dyssynchrony indices within the same subject. For data for which the normality assumption did not hold, nonparametric testing was used (systolic interventricular delay in control subjects, disease duration, and New York Heart Association class). Associations between dyssynchrony parameters and echocardiographic parameters of systolic and diastolic function were derived by linear regression. Pearson correlation coefficient was used when data were normally distributed, whereas Spearman correlation was used for bivariate analysis when data were not normally distributed. z scores for LV end-diastolic dimension were calculated with normal data obtained from our institution. To assess intraobserver and interobserver reliability of the systolic and diastolic dyssynchrony indices, 8 consecutive control studies and 8 consecutive DCM studies (16 studies in all) were reanalyzed by the same reader and by a second reader, respectively, on separate occasions for a second reread. The intraobserver and interobserver reliability are expressed as the intraclass correlation coefficient with the Cronbach’s ␣-value Figure 1. Tissue Doppler velocity curves in the 4-chamber view. Right, Tissue velocity curves. Left, Color tissue Doppler (top) and corresponding gray-scale images (bottom). Samples (5 mm) were placed during the same cardiac cycles at the mitral annulus, basal interventricular septum, midlateral LV wall, and midseptum. For clarity, in A, only the mitral annulus (yellow) and basal septum (cyan) curves are demonstrated. In B, curves from all 4 points are demonstrated in the same cardiac cycles (yellow, mitral annulus; cyan, basal septum; red, mid lateral wall; green, midseptum). The time from QRS onset to peak early diastolic velocity (E⬘) was then measured at each of the points (arrows in A). Similar curves were obtained in the orthogonal 2and 3-chamber views to yield velocity curves in 12 LV segments. The SD of time to peak early diastolic velocity (E⬘) among the 12 LV segments was used as the diastolic dyssynchrony index. A second method to assess diastolic dyssynchrony, termed the intraventricular diastolic delay, used the delay between time from QRS onset to peak early diastolic velocity (E⬘) at the mitral valve (yellow curve) and basal interventricular septum (cyan curve; arrows in A). These curves obtained from a normal control subject demonstrate near-simultaneous diastolic motion in these 4 segments. Systolic dyssynchrony was measured in a similar fashion by use of time from QRS onset to peak systolic velocity (S⬘) in the same LV segments in the same cardiac cycles. A similar curve (not demonstrated to increase clarity) may be obtained for the tricuspid annulus. The interventricular diastolic delay was defined as the time difference between time from QRS onset to peak early diastolic velocity (E⬘) at the mitral annulus and tricuspid valve. reported. Survival function was analyzed by Kaplan-Meier curves with log-rank testing for differences in survival. The hazard ratio is reported after fitting of the proportional hazards model while adjusting for disease duration and age of the patient at diagnosis. Follow-up for survival analysis was from time of diagnosis. All 52 Circ Cardiovasc Imaging July 2008 Table 1. Clinical Characteristics of 33 Children With DCM and 46 Control Subjects Clinical Feature Age, y Control Subjects (n⫽46) DCM (n⫽33) 2-Tailed P 10.6⫾5.2 8.0⫾6.3 0.056 Sex, n (%) 0.65 Males 25 (54) Females 21 (46) 17 (51) 67.2⫾6.3 28.0⫾13.5 Ejection fraction, % 16 (49) ⬍0.0001 probability values are 2 sided and considered statistically significant if ⬍0.05. The authors had full access to the data and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 Results Figure 2. Point plot depicting the diastolic dyssynchrony index in 33 children with DCM (f) and 45 control subjects (⽧). The 17-ms cutoff used to determine the presence of diastolic dyssynchrony, based on the mean⫾2 SDs in the control group, is marked. General Thirty-three patients with DCM (16 males [48%]) and 46 control subjects (25 males [54%]) were recruited. Patients were younger than control subjects, although not significantly so (8.0⫾6.3 versus 10.6⫾5.2 years, P⫽0.056; Table 1). subjects (58.5⫾29.5 versus 21.5⫾17.9 ms, P⬍0.0001). The same trend existed in DCM patients (53.5⫾51.4 versus 32.9⫾23.4 ms, P⫽0.08). Intraventricular Systolic Versus Diastolic Wall Motion in Control Subjects and in DCM Patients Correlation Between Intraventricular and Interventricular Systolic and Diastolic Mechanical Dyssynchrony Table 2 presents interventricular and intraventricular mechanical dyssynchrony in systole and diastole in control subjects and DCM patients. Diastolic wall motion was more tightly synchronized than systolic wall motion, both in normal control subjects and in DCM patients. The mean LV intraventricular systolic delay was significantly longer than the mean LV intraventricular diastolic delay in control subjects (27.3⫾24.1 versus 4.5⫾5.6 ms, P⬍0.0001) and in DCM patients (36.8⫾39.6 versus 15.1⫾17.4 ms, P⫽0.005). Likewise, the average SD of time to peak systole in 12 cardiac segments (systolic dyssynchrony index) was longer than the SD of time to peak diastole in 12 cardiac segments (diastolic dyssynchrony index), both in the control group (26.3⫾11 versus 9.1⫾3.8 ms, P⬍0.0001) and in the DCM group (42.5⫾22.1 versus 28.1⫾18.1 ms, P⫽0.002). Interventricular Systolic Versus Diastolic Wall Motion in Control Subjects and in DCM Patients The mean interventricular systolic delay was significantly greater than the interventricular diastolic delay in control Intraventricular and interventricular systolic wall motion did not correlate with diastolic wall motion either in control subjects or in DCM patients. No correlation was found between the systolic and diastolic intraventricular delay (control subjects: r⫽0.005, P⫽0.9; DCM: r⫽0.13, P⫽0.46), between the systolic and diastolic interventricular delay (control subjects: r⫽0.09, P⫽0.91; DCM: r⫽0.22, P⫽0.29), or between the SD of time to peak systole and the SD of time to peak diastole among 12 cardiac segments (control subjects: r⫽0.14, P⫽0.3; DCM: r⫽0.26, P⫽0.1). Diastolic Wall Motion in DCM Patients Versus Control Subjects The diastolic intraventricular delay was prolonged in children with DCM compared with control subjects (15.1⫾17.4 versus 4.5⫾5.6 ms, P⫽0.0009), and the SD of time to peak diastole between 12 LV segments was significantly higher in children with DCM than in control subjects (28.1⫾18.1 versus 9.1⫾3.8 ms, P⬍0.0001; Figure 2). Table 2. Systolic and Diastolic Mechanical Dyssynchrony in 33 Children With DCM and 46 Control Subjects Control Subjects DCM 2-Tailed P* LV intraventricular systolic delay, ms 27.3⫾24.1 36.8⫾39.6 0.20 LV intraventricular diastolic delay, ms 4.5⫾5.6 15.1⫾17.4 0.0009 LV systolic dyssynchrony index, ms 26.3⫾11 42.5⫾22.1 0.0003 LV diastolic dyssynchrony index, ms 9.1⫾3.8 28.1⫾18.1 ⬍0.0001 Systolic interventricular delay, ms 58.5⫾29.5 53.5⫾51.4 0.11 Diastolic interventricular delay, ms 21.5⫾17.9 32.9⫾23.4 0.02 LV intraventricular mechanical dyssynchrony Left-right interventricular mechanical dyssynchrony Friedberg et al Diastolic Dyssynchrony in Pediatric Cardiomyopathy Table 3. Correlation Between Diastolic Dyssynchrony Index and LV Functional Indices in Children With DCM Variable Correlated With the Diastolic Dyssynchrony Index Correlation Coefficient (r) P LVEDd/BSA, cm/m2 0.36 0.03 LVEDd z score 0.46 0.006 2 LVESd/BSA, cm/m 0.4 0.02 Ejection fraction, % ⫺0.3 0.09 Mitral E⬘, cm/s ⫺0.32 0.06 Mitral E/E⬘ ratio 0.2 0.22 Mitral S⬘, cm/s ⫺0.06 0.74 LVEDd indicates LV end-diastolic dimension; BSA, body surface area; and LVESd, LV end-systolic dimension. Determination of a Threshold for the Diagnosis of Diastolic Dyssynchrony Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 We used control results to establish a cutoff for determination of an abnormal diastolic dyssynchrony index. The diastolic dyssynchrony index (mean ⫹2 SD) in normal control subjects was 16.6 ms. Using this value, we selected a diastolic dyssynchrony index of ⬎17 ms as a threshold for determining the presence of diastolic dyssynchrony. This threshold defined 21 (63.6%) of 33 children with DCM as having diastolic dyssynchrony, whereas only 1 control subject had a diastolic dyssynchrony index slightly above the cutoff (19.8 ms). QRS durations of the DCM group with and without diastolic dyssynchrony are shown in Table 3; these were not different between groups. In the DCM group without diastolic dyssynchrony, 1 patient had left bundle-branch block, and 2 had right bundle-branch block. In the DCM group with diastolic dyssynchrony, 4 patients had left bundle-branch block, and 2 had right bundle-branch block. Relation Between Dyssynchrony and Echocardiographic Parameters of Cardiac Function Table 3 shows correlations between the diastolic dyssynchrony index and LV functional indices in children with DCM. The diastolic dyssynchrony index correlated modestly with LV systolic and diastolic dimensions, and there was a trend toward correlation with ejection fraction. On the other hand, there was no correlation between the SD of time to peak systole (systolic dyssynchrony index) among 12 segments and ejection fraction in DCM (r⫽⫺0.08, P⫽0.66). There was no correlation between the diastolic dyssynchrony index and mitral tissue Doppler indices (Table 3). Although in general, DCM patients without diastolic dyssynchrony had more favorable diastolic function than DCM patients with diastolic dyssynchrony, this was not statistically significant (Table 4). 53 determination of the threshold for diastolic dyssynchrony, we correlated the uncorrected diastolic dyssynchrony index with the heart rate– corrected diastolic dyssynchrony index. There was a very strong correlation between these 2 measures (DCM: r⫽0.98, P⬍0.0001; control subjects r⫽0.96, P⬍0.0001), which indicates a relatively minor effect of heart rate on the diastolic dyssynchrony index. Relation Between Dyssynchrony and Clinical Status Patients who were listed for heart transplantation or who died had more diastolic dyssynchrony than those who were free of these events (diastolic dyssynchrony index 37.9⫾20.5 versus 22.1⫾13.8 ms, P⫽0.01). The median length of transplantation-free survival of DCM patients with diastolic dyssynchrony was 4 years, versus 12 years for those without diastolic dyssynchrony, and the Kaplan-Meier survival curve of those with diastolic dyssynchrony was worse than that for those who did not have diastolic dyssynchrony, although the log-rank test did not reach statistical significance, likely because of the relatively small sample size and low event rate (hazard ratio 2.98, P⫽0.11; Figure 3).We further analyzed time-related transplantation-free survival between the 2 groups after adjustment for disease duration. This showed a similar result (hazard ratio 2.96, P⫽0.17). Age at diagnosis was not significantly associated with length of survival (hazard ratio 1.04, P⫽0.41). After controlling for age at diagnosis, the hazard ratio was 3.5 (P⫽0.11). The New York Heart Association classification of patients below the median diastolic dyssynchrony index (17 ms) was not significantly different than that of patients with a diastolic dyssynchrony index above this median value (2.5⫾0.9 versus 2.3⫾1.3, respectively, P⫽0.52; Table 5). Effect of Medications on Diastolic Dyssynchrony Because all DCM patients were receiving at least 1 kind of medication (including diuretics, spironolactone, angiotensin converting enzyme inhibitors, -blockers, and digoxin), it was difficult to determine the association between effects of medications and the degree of diastolic dyssynchrony. There were no significant associations between the proportion of patients receiving any one class of medication and the presence or absence of dyssynchrony. Interobserver and Intraobserver Reliability Correction of Diastolic Dyssynchrony Index for Heart Rate Interobserver reliability of the diastolic dyssynchrony index was higher than the interobserver reliability for the systolic dyssynchrony index in normal subjects (Cronbach’s ␣ 0.98 versus 0.69) but was equal in DCM patients (Cronbach’s ␣ 0.98 versus 0.98). Intraobserver reliability was higher for the diastolic dyssynchrony index than for the systolic dyssynchrony index in normal control subjects (Cronbach’s ␣ 0.99 versus 0.56) and in DCM patients (Cronbach’s ␣ 0.98 versus 0.8). Correction of the diastolic dyssynchrony index for heart rate by the Bazzet method (dyssynchrony index divided by the square root of the R-R interval) did not alter the results. Children with DCM still had a significantly higher diastolic dyssynchrony index than control subjects (1.0⫾0.7 versus 0.3⫾0.1, P⬍0.0001). To evaluate the effect of heart rate on LV mechanical dyssynchrony, the incoordinate wall motion of different LV segments, is an important contributor to the pathophysiology of heart failure and has been linked to worse functional status and outcomes in adult populations.13 When Discussion 54 Circ Cardiovasc Imaging July 2008 Table 4. Echocardiographic Indices of Ventricular Systolic and Diastolic Function in Normal Control Subjects and in Children With DCM With and Without Diastolic Dyssynchrony as Defined by a Diastolic Dyssynchrony Index Threshold of 17 ms Echocardiographic Index Control Subjects DCM With Diastolic Dyssynchrony DCM Without Diastolic Dyssynchrony 2-Tailed P* Ejection fraction, % 67.2⫾6.3 24.9⫾11.3 33.3⫾15.8 0.08 LVEDd, cm 4.2⫾0.7 5.2⫾1.1 5.3⫾1.5 0.87 LVEDd/BSA 3.8⫾1.3 8.1⫾4 6.3⫾3.5 0.21 LVEDd z score 6.1⫾1.7 4.8⫾2.5 0.12 LVESd, cm 2.6⫾0.5 4.5⫾0.9 4.4⫾1.5 0.84 LVESd/BSA 2.4⫾0.7 7.1⫾3.8 5.0⫾2.5 0.11 IVRT, ms 58.4⫾12.8 76.9⫾20.0 65.8⫾16.7 0.17 PV A-wave reversal velocity, cm/s 25.8⫾9.6 21.5⫾8.9 23.8⫾9.5 0.51 PV A-wave reversal duration, ms 109.2⫾35.9 97.0⫾14.9 94.3⫾33.6 0.76 PV A/mitral A velocity ratio 0.46⫾0.17 0.43⫾0.22 0.48⫾0.19 0.51 PV A/mitral A duration ratio 0.77⫾0.33 0.97⫾0.36 1.2⫾0.36 0.12 Mitral E velocity, cm/s 96.9⫾16 93.2.⫾26.2 110.7⫾21.9 0.06 Mitral A velocity, cm/s 55.3⫾15.4 52.4⫾18.7 55.1⫾23.1 0.72 Mitral E/A velocity ratio 1.9⫾0.4 2.10⫾1.0 2.3⫾0.8 0.39 158.7⫾46.4 116.7⫾32.2 105.9⫾30.5 0.38 398⫾115 300.2⫾112.9 285.7⫾148.0 0.75 Mitral valve S⬘, cm/s 9.4⫾2.9 5.2⫾2.7 6.0⫾2.1 0.38 Mitral valve E⬘, cm/s Doppler Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 Mitral valve E-wave deceleration time, ms Mitral valve filling time, ms Tissue Doppler 16.7⫾4.3 9.3⫾4.4 11.5⫾4.4 0.18 Mitral valve E/E⬘ 6.3⫾2.1 12.6⫾8.0 11⫾5.5 0.57 Septal S⬘, cm/s 7.6⫾1.7 4.9⫾1.6 5.9⫾2.4 0.23 Septal E⬘, cm/s 12⫾3.4 7.0⫾2.1 7.9⫾2.6 0.29 Septal E/E⬘ 8.2⫾2.6 13.7⫾5.3 15.3⫾5.4 0.42 LVEDd indicates LV end-diastolic dimension; BSA, body surface area; LVESd, LV end-systolic dimension; IVRT, isovolumic relaxation time; and PV, pulmonary vein. *P for comparison between DCM patients with and without diastolic dyssynchrony. tissue Doppler is used to study regional LV wall motion, mechanical dyssynchrony can be quantified by various techniques, such as the maximal motion delay between different segments or the variance (expressed as the SD) in time to Figure 3. Kaplan-Meier curve showing transplantation-free survival among DCM patients with (dashed line) and without (solid line) diastolic dyssynchrony as defined by a diastolic dyssynchrony index threshold of 17 ms. peak myocardial tissue velocity between multiple ventricular segments.13 Although the bulk of research both in adults1,2,14 –16 and in children3,17 relating to mechanical dyssynchrony has focused on systolic wall motion, recent findings indicate that diastolic wall-motion abnormalities are prevalent in adults with heart failure, both with and without systolic dysfunction. 6,8,9 The present study is the first to investigate diastolic mechanical dyssynchrony in children with DCM and the first to explore its relation to clinical status in this group of patients. The major findings of the present study were as follows: (1) LV diastolic wall motion is highly synchronized in normal children; (2) LV intraventricular diastolic mechanical dyssynchrony is common in pediatric DCM; (3) an intraventricular diastolic dyssynchrony index of 17 ms is a reasonable threshold to define the presence of diastolic mechanical dyssynchrony in children; and (4) DCM patients with worse transplant-free survival have more diastolic dyssynchrony and conversely, those with diastolic dyssynchrony appear to have a worse survival than patients without diastolic dyssynchrony. Using our normal control results as a reference, we found that diastolic mechanical dyssynchrony is common in pediatric DCM and occurs at a rate similar to the 60% rate of Friedberg et al Diastolic Dyssynchrony in Pediatric Cardiomyopathy 55 Table 5. Clinical Features of DCM Patients With and Without Diastolic Dyssynchrony Clinical Feature Age, y DCM With Diastolic Dyssynchrony (n⫽21) DCM Without Diastolic Dyssynchrony (n⫽11) 2-Tailed P 7.3⫾5.9 9.5⫾7.0 0.33 QRS duration, ms 87.5⫾15.7 89.3⫾28.4 0.85 Mean NYHA class 2.3⫾1.3 2.5⫾0.9 0.52 % Patients who received transplants or died 47 27 0.45 % Patients admitted to hospital with worsening failure 38 36 0.80 1.9⫾2.9 2.9⫾4.5 0.91 Time between diagnosis and echocardiography (illness duration at time of evaluation), y Cause of DCM Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 Idiopathic 13 5 ALCAPA 2 0 Myocarditis 1 0 Familial 1 1 Metabolic 1 2 Other systemic disease 1 1 Anthracycline 1 2 Arrhythmogenic 1 0 ALCAPA indicates anomalous left coronary artery from pulmonary artery; DCM, dilated cardiomyopathy. diastolic dyssynchrony seen in adult patients with systolic and diastolic heart failure.8 The presence of diastolic wallmotion abnormalities is important because DCM patients who later died or were listed for heart transplantation had significantly more diastolic dyssynchrony than did survivors. The actuarial survival of DCM patients with diastolic dyssynchrony appeared worse than that of patients who had synchronous diastolic motion, and although this did not reach the statistical cutoff to determine significance, likely because of the small sample size and low event rate, we believe that this is clinically significant and important nonetheless. Statistical significance should be verified with further study on a larger group of patients. The present findings are in keeping with diastolic dysfunction being an important prognostic factor in heart failure4; however, although the present results indicate a worse clinical outcome for patients with diastolic dyssynchrony, they do not show whether this is causally related or a manifestation of overall disease severity. We did find that the diastolic dyssynchrony index correlated with LV end-diastolic and end-systolic dimensions. These are important components of ventricular function and remodeling and are also important prognostic indices. Ventricular reverse remodeling has also been used as a primary outcome measure in several trials that have investigated the association between mechanical dyssynchrony and response to cardiac resynchronization therapy,18,19 and in conjunction with that, the present results may suggest that in children with decreased systolic function, there is an important association between diastolic dyssynchrony, ventricular remodeling, and clinical prognosis. Conversely, systolic dyssynchrony did not affect these pa- rameters. Diastolic dyssynchrony may adversely affect ventricular function by adversely affecting filling dynamics,8,20,21 compromising coronary perfusion and ventricular function.22 In DCM, there is a disproportionate shortening of diastolic time over and above that related to an increase in heart rate,23 and in the present study, even when corrected for heart rate, diastolic dyssynchrony was significantly higher in DCM than in control subjects. However, the E/E⬘ ratio and other echocardiographic parameters of diastolic function were similar between patients with and without diastolic dyssynchrony, and the mechanism whereby diastolic dyssynchrony impacts ventricular function remains to be further elucidated. Diastolic Versus Systolic Motion in Normal Children and in Children With DCM Measurement of diastolic dyssynchrony was easily achieved with good interobserver and intraobserver reliability. In the present study, diastolic wall motion was highly synchronized as compared with systolic wall motion both in control subjects and in DCM. These results differ somewhat from those found in a previous study in the adult population, in which the diastolic dyssynchrony index was very similar to the systolic dyssynchrony index both in normal subjects and in patients with heart failure.9 In addition, the diastolic dyssynchrony index of 17 ms we determined as a threshold for diagnosis of diastolic dyssynchrony was lower than that found in the study by Yu and colleagues.9 Although this may be the result of shorter cardiac intervals in children, leading to a smaller SD, it may also be the result of greater diastolic synchrony in children than in adults, possibly related to the proportionately shorter diastolic period in children.24 56 Circ Cardiovasc Imaging July 2008 Consistent with previous findings from studies in adults,9 we found no relation between systolic and diastolic wall motion either in normal control subjects or in DCM patients. This implies that the mechanisms that lead to systolic and diastolic mechanical dyssynchrony are distinct from one another. We did not investigate the reasons for the lack of correlation between systolic and diastolic dyssynchrony, and this requires further study. 2. 3. 4. Study Limitations Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 This was a cross-sectional study of echocardiographic assessment, with inherent limitations. In our institution, children with DCM do not routinely undergo diagnostic cardiac catheterization, and therefore, we did not have data relating to filling pressures or measurements of the time constant of pressure decay in the LV (). None of the patients studied underwent cardiac resynchronization therapy, and therefore, it was not possible to study the effect of cardiac resynchronization therapy on diastolic dyssynchrony. Although we only measured longitudinal motion, it is possible that radial and circumferential diastolic dyssynchrony is important, and this requires further study. Future Implications Currently, there are no data on the management of diastolic dyssynchrony in children with DCM. Wang et al8 found that medical therapy, including diuretics, -blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, improved diastolic dyssynchrony in adults with cardiomyopathy and decreased filling pressures, but they did not investigate which of these medications brought about these changes. Schuster et al6 found in adults that cardiac resynchronization therapy reduced diastolic dyssynchrony, albeit with lesser effect than systolic dyssynchrony. Others have found that pacing improves diastolic ventricular–ventricular interactions.25,26 Given the present finding that diastolic dyssynchrony is common in children with DCM, and given that the outcome of children with symptomatic DCM is poor,27 the effects of various interventions on diastolic dyssynchrony and function need to be investigated as an alternative avenue of therapy to that focused only on improving systolic function. Conclusions 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. LV intraventricular diastolic mechanical dyssynchrony is common in children with DCM and is worse in children who later experience heart transplantation or death. The presence of diastolic dyssynchrony in pediatric DCM appears to be associated with a worse clinical outcome. Acknowledgments We thank Cameron Slorach and Cheryl Fackoury for their contribution in performing echocardiography. 16. 17. 18. Disclosures None. References 1. Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts 19. response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834 –1840. Breithardt OA, Stellbrink C, Kramer AP, Sinha AM, Franke A, Salo R, Schiffgens B, Huvelle E, Auricchio A. Echocardiographic quantification of left ventricular asynchrony predicts an acute hemodynamic benefit of cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40:536 –545. Friedberg MK, Silverman NH, Dubin AM, Rosenthal DN. Mechanical dyssynchrony in children with systolic dysfunction secondary to cardiomyopathy: a Doppler tissue and vector velocity imaging study. J Am Soc Echocardiogr. 2007;20:756 –763. Xie GY, Berk MR, Smith MD, Gurley JC, DeMaria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol. 1994;24:132–139. Hansen A, Haass M, Zugck C, Krueger C, Unnebrink K, Zimmermann R, Kuebler W, Kuecherer H. Prognostic value of Doppler echocardiographic mitral inflow patterns: implications for risk stratification in patients with chronic congestive heart failure. J Am Coll Cardiol. 2001;37:1049 –1055. Schuster I, Habib G, Jego C, Thuny F, Avierinos JF, Derumeaux G, Beck L, Medail C, Franceschi F, Renard S, Ferracci A, Lefevre J, Luccioni R, Deharo JC, Djiane P. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2250 –2257. Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88 –96. Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, Fung JW. Diastolic and systolic asynchrony in patients with diastolic heart failure: a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105. Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54 – 60. Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684 – 688. Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66 –73. Gorcsan J III, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting: a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, Spinelli J, Halperin H, McVeigh E, Kass DA. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106: 1760 –1763. Penicka M, Bartunek J, De Bruyne B, Vanderheyden M, Goethals M, De Zutter M, Brugada P, Geelen P. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation. 2004;109:978 –983. Yu CM, Bax JJ, Monaghan M, Nihoyannopoulos P. Echocardiographic evaluation of cardiac dyssynchrony for predicting a favourable response to cardiac resynchronisation therapy. Heart. 2004;90(suppl 6):vi17–vi-22. Friedberg MK, Silverman NH, Dubin AM, Rosenthal DN. Right ventricular mechanical dyssynchrony in children with hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2007;20:1073–1079. Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238 –1240. Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, Kong SL, Lin H, Zhang Y, Sanderson JE. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005;45:677– 684. Friedberg et al Diastolic Dyssynchrony in Pediatric Cardiomyopathy 20. Bonow RO, Vitale DF, Bacharach SL, Frederick TM, Kent KM, Green MV. Asynchronous left ventricular regional function and impaired global diastolic filling in patients with coronary artery disease: reversal after coronary angioplasty. Circulation. 1985;71:297–307. 21. Bonow RO, Vitale DF, Maron BJ, Bacharach SL, Frederick TM, Green MV. Regional left ventricular asynchrony and impaired global left ventricular filling in hypertrophic cardiomyopathy: effect of verapamil. J Am Coll Cardiol. 1987;9:1108 –1116. 22. Gibson DG, Prewitt TA, Brown DJ. Analysis of left ventricular wall movement during isovolumic relaxation and its relation to coronary artery disease. Br Heart J. 1976;38:1010 –1019. 23. Friedberg MK, Silverman NH. Cardiac ventricular diastolic and systolic duration in children with heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:101–105. 57 24. Spitaels S, Arbogast R, Fouron JC, Davignon A. The influence of heart rate and age on the systolic and diastolic time intervals in children. Circulation. 1974;49:1107–1115. 25. Bleasdale RA, Turner MS, Mumford CE, Steendijk P, Paul V, Tyberg JV, Morris-Thurgood JA, Frenneaux MP. Left ventricular pacing minimizes diastolic ventricular interaction, allowing improved preload-dependent systolic performance. Circulation. 2004;110:2395–2400. 26. Morris-Thurgood JA, Turner MS, Nightingale AK, Masani N, Mumford C, Frenneaux MP. Pacing in heart failure: improved ventricular interaction in diastole rather than systolic re-synchronization. Europace. 2000; 2:271–275. 27. Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. J Am Med Assoc. 2006;296:1867–1876. CLINICAL PERSPECTIVE Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 Left ventricular mechanical dyssynchrony is generally thought of as a systolic phenomenon and appears to be an important contributor to the pathophysiology of heart failure. It has been associated with worse functional status and outcomes in adult populations with cardiomyopathy. The significance of diastolic dyssynchrony has not been fully evaluated, particularly in the pediatric population. This is the first study investigating diastolic mechanical dyssynchrony in children with dilated cardiomyopathy. This report, using tissue Doppler imaging, establishes normal values for diastolic dyssynchrony in pediatric patients and shows that diastolic dyssynchrony is relatively common among pediatric patients with dilated cardiomyopathy. We investigate the relation between diastolic mechanical dyssynchrony, ventricular function, and clinical outcomes and show that the presence of diastolic dyssynchrony may have implications for length of transplantation-free survival. These results should stimulate further research into diastolic mechanical dyssynchrony in children with heart failure and allow investigation of possible treatment modalities to address it. Left Ventricular Diastolic Mechanical Dyssynchrony and Associated Clinical Outcomes in Children With Dilated Cardiomyopathy Mark K. Friedberg, Susan L. Roche, Arshiya F. Mohammed, Mervin Balasingam, Eshetu G. Atenafu and Paul F. Kantor Downloaded from http://circimaging.ahajournals.org/ by guest on May 12, 2017 Circ Cardiovasc Imaging. 2008;1:50-57 doi: 10.1161/CIRCIMAGING.108.782086 Circulation: Cardiovascular Imaging is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2008 American Heart Association, Inc. All rights reserved. Print ISSN: 1941-9651. Online ISSN: 1942-0080 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circimaging.ahajournals.org/content/1/1/50 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation: Cardiovascular Imaging can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation: Cardiovascular Imaging is online at: http://circimaging.ahajournals.org//subscriptions/