* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Infective Endocarditis
Remote ischemic conditioning wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Aortic stenosis wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Coronary artery disease wikipedia , lookup
Jatene procedure wikipedia , lookup
Pericardial heart valves wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Artificial heart valve wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiac surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Rheumatic fever wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
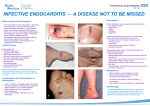
Infective Endocarditis Faculty of Medicine University of Brawijaya Malang INTRODUCTION The term ‘bacterial endocarditis’ has been replaced by ‘Infective endocarditis’ (IE) since fungi are also involved as causative pathogens IE is an uncommon but lifethreatening infection. If the diagnosis is delayed or appropriate therapeutic measures postpone, mortality is still high JADA, Vol. 138, 2007 European Heart Journal (2004) 00, 1-37 Guidelines AHA, Circulation. 2007;115:&NA;-. INTRODUCTION If untreated Infective Endocarditis (IE) is a fatal disease. Major diagnostic (first of all echocardiography) and therapeutic progress (mainly surgery during active IE) have contributed to some prognostic improvement. In this respect, it is of utmost importance that: – IE is considered early in every patients with fever or septicaemia and cardiac murmurs. – Echocardiography is applied without delay in suspected IE. – Cardiologist, microbiologists and cardiac surgeons cooperate closely if IE is suspected or definite. INTRODUCTION Recent data suggest it may be increasing, 1. In industrialized nations, patients are living longer 2. There is an increase in nosocomial infections 3. Intravenous drug use has increased in industrialized societies 4. Increasing application of cardiac surgery has provided new substrates for endocardial infection 5. The increased use of indwelling intravascular lines and implantable devices 6. the increased application of echocardiography Cardiol Clin 21 (2003) 159–166 DEFINITION IE is an endovascular, microbial infection of intracardiac structures facing the blood including infections of the large intrathoracic vesseis and of intracardiac foreign bodies. The early characteristic lesion is a variably sized vegetation, although destruction, ulceration or abscess formation may be seen earlier by echocardiography. Definition Infectious Endocarditis (IE): an infection of the heart’s endocardial surface Classified into four groups: – Native Valve IE – Prosthetic Valve IE – Intravenous drug abuse (IVDA) IE – Nosocomial IE INFECTIVE ENDOCARDITIS Characterized by inflammation or infection two major predisposing factors: – susceptible cardiac or vascular substrate lesions associated with high-velocity flow, jet impact and focal increases in the rate of shear – source of bacteremia Further Classification Acute – Affects normal heart valves – Rapidly destructive – Metastatic foci – Commonly Staph. – If not treated, usually fatal within 6 weeks Subacute – Often affects damaged heart valves – Indolent nature – If not treated, usually fatal by one year Pathophysiology 1. Turbulent blood flow disrupts the endocardium making it “sticky” 2. Bacteremia delivers the organisms to the endocardial surface 3. Adherence of the organisms to the endocardial surface 4. Eventual invasion of the valvular leaflets Proposed scheme for the pathogenesis of infective endocarditis Mandell, Bennett, & Dolin: Principles and Practice of Infectious Diseases, 6th ed Epidemiology Incidence difficult to ascertain and varies according to location Much more common in males than in females May occur in persons of any age and increasingly common in elderly Mortality ranges from 20-30% Risk Factors Intravenous drug abuse Artificial heart valves and pacemakers Acquired heart defects – Calcific aortic stenosis – Mitral valve prolapse with regurgitation Congenital heart defects Intravascular catheters Infecting Organisms Common bacteria – S. aureus – Streptococci – Enterococci Not so common bacteria – Fungi – Pseudomonas – HACEK HACEK organisms • Hemophilus, Actinobacillus, • • • • Cardiobacterium, Eikenella, Kingella Gram negative inhabitants of the upper airways. Large vegetations, high likelihood of embolization. Slow growing: hold cultures for 3 weeks. Traditionally sensitive to beta lactams, now some produce beta lactamase. Symptoms Acute – High grade fever and chills – SOB (shortness of breath) – Arthralgias/ myalgias – Abdominal pain – Pleuritic chest pain – Back pain Subacute – – – – – – Low grade fever Anorexia Weight loss Fatigue Arthralgias/ myalgias Abdominal pain The onset of symptoms is usually ~2 weeks or less from the initiating bacteremia Signs Fever Heart murmur Nonspecific signs – petechiae, subungal or “splinter” hemorrhages, clubbing, splenomegaly, neurologic changes More specific signs - Osler’s Nodes, Janeway lesions, and Roth Spots Petechiae 1. Nonspecific 2. Often located on extremities or mucous membranes dermatology.about.com/.../ blpetechiaephoto.htm Photo credit, Josh Fierer, M.D. medicine.ucsd.edu/clinicalimg/ Eye-Petechiae.html Harden Library for the Health Sciences www.lib.uiowa.edu/ hardin/ md/cdc/3184.html Splinter Hemorrhages 1. Nonspecific 2. Nonblanching 3. Linear reddish-brown lesions found under the nail bed 4. Usually do NOT extend the entire length of the nail Osler’s Nodes American College of Rheumatology webrheum.bham.ac.uk/.../ default/pages/3b5.htm www.meddean.luc.edu/.../ Hand10/Hand10dx.html 1. More specific 2. Painful and erythematous nodules 3. Located on pulp of fingers and toes 4. More common in subacute IE Janeway Lesions 1. More specific 2. Erythematous, blanching macules 3. Nonpainful 4. Located on palms and soles The Essential Blood Test Blood Cultures – Minimum of three blood cultures1 – Three separate venipuncture sites – Obtain 10-20mL in adults and 0.5-5mL in children2 Positive Result – Typical organisms present in at least 2 separate samples – Persistently positive blood culture (atypical organisms) Two positive blood cultures obtained at least 12 hours apart Three or a more positive blood cultures in which the first and last samples were collected at least one hour apart Additional Labs CBC (complete blood count) ESR (erythrocyte sedimentation rate) and CRP (C reactive protein) Complement levels (C3, C4, CH50) RF (rheumatoid factors) Urinalysis Baseline chemistries and coagulations Imaging Chest x-ray – Look for multiple focal infiltrates and calcification of heart valves EKG – Rarely diagnostic – Look for evidence of ischemia, conduction delay, and arrhythmias Echocardiography Indications for Echocardiography Transthoracic echocardiography (TTE) – First line if suspected IE – Native valves Transesophageal echocardiography (TEE) – Prosthetic valves – Intracardiac complications – Inadequate TTE – Fungal or S. aureus or bacteremia Making the Diagnosis Pelletier and Petersdorf criteria (1977) – Classification scheme of definite, probable, and possible IE – Reasonably specific but lacked sensitivity Von Reyn criteria (1981) – Added “rejected” as a category – Added more clinical criteria – Improved specificity and clinical utility Duke criteria (1994) – Included the role of echocardiography in diagnosis – Added IVDA as a “predisposing heart condition” Modified Duke Criteria Definite IE – Microorganism (via culture or histology) in a valvular vegetation, embolized vegetation, or intracardiac abscess – Histologic evidence of vegetation or intracardiac abscess Possible IE – 2 major – 1 major and 3 minor – 5 minor Rejected IE – Resolution of illness with four days or less of antibiotics CRITERIA THAT SHOULD RAISE SUSPICION OF IE High clinical suspicion (urgent indication for echocardiographic screening and possibly hospital admission) – – – – – New valve lesion/(regurgitant) murmur Embolic events of unknown origin (esp. cerebral and renal infarction) Sepsis of unknown origin Haematuria, glomerulonephritis, and suspected renal infarction “Fever “ plus Prosthetic material inside the heart Other high predispositions of IE Newly developed ventricular arrhythmias or conduction disturbances First manifestation of chronic heart failure Positive blood cultures (if the organism identified is typical for NVE/PVE) Cutaneous (Osler, janeway) or ophthalmic (roth) manifestations Multifocal/rapid changing pulmonary infiltrations (right heart IE) Peripheral abscesses (renal, spienic, spine) of unknown origin Predisposition and recent diagnostic/therapeutic interventions known to result in significant bacteraemia Low Clinical Suspicion – Fever plus none of the above ECHOCARDIOGRAPHY Any patient suspected of having Native Valve Endocarditis (NVE) by clinical criteria should be screened by Transthoracic Echocardiography (TTE). When images are of good quality and prove to be negative and there is only a low clinical suspicion of IE, endocarditis is unlikely and other diagnosis are to be considered. If suspicion of IE is high, TransEsophageal Echocardiography (TEE) should be performed in all TTE-negative cases, in suspected Prosthetic Valve Endocarditis (PVE), and if TTE is positive but complications are suspected or likely and before cardiac surgery during active IE. If TEE remains negative and there is still suspicion, it should be repeated within one week. A repeatedly negative study should virtually exclude the diagnosis. Three echocardiographic findings are considered to be major critetria in the diagnosis of IE: – A mobile, echodense mass attached to the valvular or the mural endocardium or to implanted prosthetic material – Demonstration of abscesses or fistulas – A new dehiscence of a valve prosthesis, especially when occurring late after implantation Treatment Parenteral antibiotics – High serum concentrations to penetrate vegetations – Prolonged treatment to kill dormant bacteria clustered in vegetations Surgery – Intracardiac complications Surveillance blood cultures ANTIMICROBIAL THERAPY If initiation of antimicrobial therapy is urgent, empiric antibiotic treatment can be started thereafter (blood culture) In all other cases it is recommended to postpone therapy until blood cultures become positive. Previous short term antibiotic discontinue for at least 3 day before taking blood cultures. Previous long term antibiotic treatment discontinue for 6 - 7 days. ESC guideline; European Heart J 2004 2 weeks regimen (combination) has similar cure rates to 4 week regimen 4 week regimen (monotherapy) preferred in – patients >65 yo – with 8th cranial nerve impairment – renal dysfunction – Cardiac/extracardiac abscess Vancomycin only for patients not tolerate to penicillin / ceftriaxone For combination antibiotics given at same time or close together to increase synergistic effect IE IN INTRAVENOUS DRUG USER (IVDU) Most common: S aureus *, ** MRSA had been emerging (60-70% in Europe)** Other organisms: P aeruginosa, Candida, enterococci, streptococci *, ** Polymicrobial infection 5-10% ** * AHA guidelines IE. Circulation 2005;111;e394-e433 ** ESC guidelines Infective Endocarditis 2004 ANTIBIOTIC PROPHYLAXIS Background for prophylaxis: – Bacteremia causes endocarditis – Viridans group streptococci are part of normal oral flora, and enterococci are part of normal GI and GU tract flora – These microorganisms were usually susceptible to antibiotics recommended for prophylaxis – Antibiotic prophylaxis prevents viridans group streptococcal or enterococcal experimental endocarditis in animals AHA guideline: Prevention of Infective Endocarditis. Circulation 2007;115 – Large number of poorly documented case reports implicated a dental procedure as a cause of IE – In some cases, there was a temporal relationship between a dental procedure and the onset of symptoms of IE – The risk of significant adverse reactions to an antibiotic is low in an individual patient – Morbidity and mortality from IE are high. Cardiac condition in Which Antimicrobial Prophylaxis is Indicated High Risk – – – – Prosthetic heart valves Complex congenital cyanotic heart diseases Previous infective endocarditis Surgically constructed systemic or pulmonary conduits Moderate Risk – Acquired valvular heart disease – Mitral valve prolapse with valvular regurgitation or severe valve thickening – Non-cyanotic congenital heart diseases (except for secundum type Atrial Septal Defect) including bicuspid aortic valves – Hypertrophic cardiomyopathy Predisposing diagnostic and therapeutic interventions Procedure which may cause bacteraemia and for which antimicrobial prophylaxis is recommended Diagnostic and therapeutic interventions likely to produce bacteraemia – – – – – – – – – – – Bronchoscopy (rigid instrument) Cystoscopy during urinary tract infection Biopsy of urinary tract/prostate Dental procedures with the risk of gingival/mucosal trauma Tonsillectomy and adenoidectomy Oesophageal dilatation/ sclerotherapy Instrumentation of obstructed biliary tracts Transurethral resection of prostate Urethral instrumentation/ dilation Lithotripsy Gynaecologic procedures in the presence of infection New from AHA for IE prophylaxis Bacteremia from daily activities (chewing food, tooth brushing and flossing, use of wooden toothpicks, use of water irrigation devices) is much more likely to cause IE than a dental procedure Extremely small number of IE might be prevented by antibiotic prophylaxis, even if prophylaxis is 100% effective AHA guideline: Prevention of Infective Endocarditis. Circulation 2007;115 Limit prophylaxis only to conditions with high adverse outcome from endocarditis Maintenance of optimal oral health and hygiene may reduce the incidence of bacteremia from daily activities and is more important than prophylactic antibiotics AHA guideline: Prevention of Infective Endocarditis. Circulation 2007;115 Complications Four etiologies – Embolic – Local spread of infection – Metastatic spread of infection – Formation of immune complexes – glomerulonephritis and arthritis Embolic Complications Occur in up to 40% of patients with IE Predictors of embolization – Size of vegetation – Left-sided vegetations – Fungal pathogens, S. aureus, and Strep. Bovis Incidence decreases significantly after initiation of effective antibiotics Embolic Complications Stroke Myocardial Infarction – Fragments of valvular vegetation or vegetation-induced stenosis of coronary ostia Ischemic limbs Hypoxia from pulmonary emboli Abdominal pain (splenic or renal infarction) Septic Pulmonary Emboli http://www.emedicine.com/emerg/topic164.htm Septic Retinal Embolus Local Spread of Infection Heart failure – Extensive valvular damage Paravalvular abscess (30-40%) – Most common in aortic valve, IVDA, and S. aureus – May extend into adjacent conduction tissue causing arrythmias – Higher rates of embolization and mortality Pericarditis Fistulous intracardiac connections Local Spread of Infection Acute S. aureus IE with perforation of the aortic valve and aortic valve vegetations. Acute S. aureus IE with mitral valve ring abscess extending into myocardium. Metastatic Spread of Infection Metastatic abscess – Kidneys, spleen, brain, soft tissues Meningitis and/or encephalitis Vertebral osteomyelitis Septic arthritis Poor Prognostic Factors Female S. aureus Vegetation size Aortic valve Prosthetic valve Older age Diabetes mellitus Low serum albumen Apache II score Heart failure Paravalvular abscess Embolic events Summary IVDA and the elderly are at greatest risk of developing IE. The signs and symptoms of IE are nonspecific and varied. A thorough but timely evaluation (including serial blood cultures, adjunct labs, and an echo) is crucial to accurately diagnose and treat IE. Beware of life-threatening complications. THANK YOU ACUTE PERICARDITIS Faculty of Medicine Universitas Brawijaya Malang Incidence Exact incidence and prevalence are unknown Diagnosed in 0.1% of hospitalized patients and 5% of patients admitted for non-acute MI chest pain Observational study: 27.7 cases/100,000 population/year Etiology: Can be Tricky. . . Standard diagnostic evaluations are oftentimes relatively low yield One series elucidated a cause in only 16% of patients Leading possibilities: – Neoplasia – Tuberculosis – Non-tuberculous infection – Rheumatic disease Initial clinical and echocardiographic evaluation of patients with suspected acute pericarditis Diagnostic Criteria Chest pain: anterior chest, sudden onset, pleuritic; may decrease in intensity when leans forward, may radiate to one or both trapezius ridges Pericardial friction rub: most specific, heard best at LSB (Left sternal border) EKG changes: new widespread ST elevation or PR depression Pericardial effusion: absence of does not exclude diagnosis of pericarditis Supporting signs/symptoms: Elevated ESR (erythrocyte sedimentation rate), CRP (C reactive protein) Fever Leukocytosis EKG Electrocardiogram in acute pericarditis showing diffuse upsloping ST segment elevations seen best here in leads II, III, aVF, and V2 to V6. There is also subtle PR segment deviation (positive in aVR, negative in most other leads). ST segment elevation is due to a ventricular current of injury associated with epicardial inflammation; similarly, the PR segment changes are due to an atrial current of injury which, in pericarditis, typically displaces the PR segment upward in lead aVR and downward in most other leads. Pericardial Effusion Cardiomegaly due to a massive pericardial effusion. At least 200 mL of pericardial fluid must accumulate before the cardiac silhouette enlarges. Tests EKG CXR PPD ANA HIV Blood cultures Urgent echocardiogram if evidence of pericardial effusion Not necessary: Viral studies b/c yield is low and management is not altered Treatment NSAIDs + PPI Aspirin (2-5 g/day) Ibuprofen (300-800 mg q6-8H)* Ketorolac Theoretical concern that anti-platelet agents promote development of hemorrhagic pericardial effusion has not been substantiated Colchicine (0.5-1 mg/day) : may prevent recurrence Glucocorticoids (prednisone 1 mg/kg/day): ? increased rate of complications. Should be restricted to: Acute pericarditis due to connective tissue disease Autoreactive (immune-mediated) pericarditis Uremic pericarditis *NSAID of choice unless associated with acute MI, where all non-ASA NSAIDs should be avoided Prognosis for acute idiopathic pericarditis Good long-term prognosis Cardiac tamponade is rare, but up to 70% in cases with specific etiologies (eg. Neoplastic, tuberculous, purulent) Constrictive pericarditis occurs in about 1% of patients 15-30% of patients not treated with colchicine develop either recurrent or incessant disease Recurrent Pericarditis Exact recurrence rate unknown Most cases considered to be autoimmune Risk Factors: – Lack of response to aspirin or other NSAID – Glucocorticoid therapy – Inappropriate pericardiotomy – Creation of a pericardial window For some patients, symptoms can only be controlled with steroidal therapy Autoreactive Pericarditis: diagnostic criteria Pericardial fluid revealing >5000/mm3 mononuclear cells or antisarcolemmal antibodies Inflammation in epicardial/endomyocardial biopsies by >14 cells/mm2 Exclusion of active viral infection both in pericardial effusion and endocardial/epicardial biopsies Exclusion of tuberculosis, borrelia burgdorferi, chlamydia pneumoniae and other bacterial infection Absence of neoplastic infiltration in effusion and biopsy samples Exclusion of systemic, metabolic disorders and uremia Treatment Aspirin NSAIDs Colchicine: can reduce or eliminate need for glucocorticoids Glucocorticoids: should be avoided unless required to treat patients who fail NSAID and colchicine therapy Many believe that prednisone may perpetuate recurrences Intrapericardial glucocorticoid therapy: sx improvement and prevention of recurrence in 90% of patients at 3 months and 84% at one year Other immunosuppression Azothoprine (75-100 mg/day) Cyclophosphamide Mycophenolate: anecdotal evidence only Methotrexate: limited data IVIG: limited data Pericardiectomy: To avoid poor wound healing, recommended to be off prednisone for one year. Reserved for the following cases: If >1 recurrence is accompanied by tamponade If recurrence is principally manifested by persistent pain despite an intensive medical trial and evidence of serious glucocorticoid toxicity