* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download B Cell Development
Homeostasis wikipedia , lookup
Embryonic stem cell wikipedia , lookup
Cell culture wikipedia , lookup
Somatic cell nuclear transfer wikipedia , lookup
List of types of proteins wikipedia , lookup
Microbial cooperation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Artificial cell wikipedia , lookup
Human genetic resistance to malaria wikipedia , lookup
State switching wikipedia , lookup
Neuronal lineage marker wikipedia , lookup
Stem-cell therapy wikipedia , lookup
Induced pluripotent stem cell wikipedia , lookup
Cell theory wikipedia , lookup
Chimera (genetics) wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
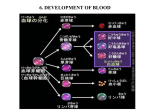
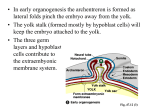
Lineages and Stem Cells: B-cell development The Circulatory System The circulatory system consists of the heart (to pump blood),the blood vessels (to transport blood) and the blood itself. All of these must develop concurrently in the early embryo to allow a functioning circulatory system What is blood? Mammalian blood is 45% red blood cells, 1% white blood cells and the remainder is plasma (the liquid portion containing various dissolved proteins). Why do we need blood cells? 1.) Red blood cells carry oxygen to tissues and carbon dioxide to lungs (or to the placenta prior to birth) 2.) Platelets are crucial for blood clotting, which prevents excess bleeding if there is a breach in the integrity of a blood vessel 3.) White blood cells fight infection. The first two functions need to be peformed at an early stage of embryonic development (as soon as blood vessels form), but the last one is only needed mainly after birth. So not all blood cell types are produced at the same time in the embryo When is blood first made in an embryo? Early human (before 16 days gestation) and mouse embryos (before 7 days gestation) do not have blood. Instead the embryo obtains all the required nutrients and oxygen from simple diffusion. Waste products also diffuse out of the embryo into the mother’s circulation. Once the embryo reaches a large enough size, simple diffusion is not sufficient to bring nutrients or remove waste products necessitating the development of a circulatory system and blood. 5 days 6 days 7 days 8 days 12 days Hematopoiesis Hematopoiesis means forming blood Hematopoiesis has discrete stages in embryonic development In mammals, blood cell development takes place in four main organs - yolk sac, placenta, fetal liver and bone marrow and two discrete stages - primitive and definitive Places of Hematopoiesis Note: The AGM is part of the early embryonic body, described later Primitive Versus Definitive Erythropoiesis Primitive erythropoiesis – Red blood cells have nuclei Primitive erythropoiesis occurs in early embryos Definitive erythropoiesis Red blood cells DO NOT have nuclei Definitive erythropoiesis occurs in later embryos and adults The Hemangioblast Studies have demonstrated that there are cells in the early embryo that have the potential to develop into both blood cells and vascular endothelial cells. These cells are termed hemangioblasts and are derived from mesodermal cells. Hemangioblasts give rise to hematopoietic stem cells that then form all the different types of blood cells Hematopoietic Stem Cells The HSC All blood cells are derived from hematopoietic stem cells (HSC). Stem cell can proliferate to replace themselves and to give rise to more committed progeny. Lots of red blood cells and platelets are needed. Many fewer of the other cell types are required Yolk Sac Hematopoiesis Blood and a circulatory system need to start forming beginning around day 18-20 of human development and around day 7-8 of mouse development At this stage, the embryos are still very tiny, so there is not much room to generate blood Therefore, the first blood cells to form are made outside the embryo in a membrane Yolk Sac called the yolk sac Embryo Yolk Sac Hematopoiesis (blood islands) In the yolk sac, the first place of hematopoiesis, undifferentiated mesenchymal cells differentiate to clusters of hemangioblast cells. The hemangioblasts further differentiate into endothelial cells and primitive blood cells. This is the first “blood vessel” like structure in the embryo. Fetal Liver Hematopoiesis When the embryo is bigger and more developed, hematopoiesis switches from the yolk sac to the fetal liver. This occurs around day 30-40 of human development and around day 10-11 of mouse development. The AGM region Derived from mesoderm that is associated with the internal organs of the body. This mesoderm lies close to and contributes to the development of the aorta, the early kidney (mesonephros) and the gonad. Stem cells in the AGM Hematopoietic stem cells from the yolk sac or the aorta-gonad-mesonephros (AGM) region of the embryo migrate to the fetal liver to give rise to fetal liver hematopoiesis Another view of AGM Blood Formation Fetal Liver Hematopoiesis Colonized by definitive hematopoietic stem cells derived from the AGM and possibly also from the yolk sac blood islands. All blood cell types (except T cells) can differentiate in the fetal liver. Fetal liver cells Neutrophil (n) Placental hematopoiesis The discovery of the placenta as a major source of embryonic blood cells came later than the other tissues and is less well-understood than yolk sac, fetal liver or bone marrow hematopoiesis. Hematopoiesis in the placenta overlaps with yolk sac and fetal liver hematopoiesis. Source of blood cells in the placenta Appear to derive from cluster of hematopoietic stem cells associated with the fetal vessels on the fetal side of the placenta The placenta may have more early blood cell progenitors than the fetal liver Source of GEMM GEMM are types of blood cell colonies derived from early hematopoietic progenitors, while BFU-E and GM are colonies derived from more committed progenitors Bone Marrow Hematopoiesis In adult humans and mice, hematopoiesis mainly takes place in the bone marrow. The figure above shows the main sites of hematopoiesis in adult humans (red bones are the ones with highest blood cell production) Timing of bone marrow hematopoiesis Bone marrow hematopoiesis begins in late in gestation (day 17-18 until birth in mice, week 22birth in humans), because the bones have not developed sufficiently before this time to support hematopoiesis Bone marrow hematopoiesis continues throughout adult life, but hematopoiesis can also occur in the liver or spleen if the bone marrow is not producing enough blood cells The Bone Marrow Hematopoiesis Colonized late in embryogenesis by definitive hematopoietic stem cells derived from the fetal liver (and placenta?). All blood cell types (except T cells) can differentiate in the bone marrow. Generalities of Hematopoiesis No matter which organ (yolk sac, placenta, fetal liver or bone marrow, hematopoietic stem cells and their more differentiated progeny need a proper environment to grow and differentiate. This includes proper cell-cell contacts, proper cytokines and growth factors and other additional requirements for particular blood cell lineages These specific requirements can explain, at least in part, why blood cannot form anywhere in an embryo, but is localized to very specific regions Various types of cytokines and growth factors are also required for hematopoiesis to occur Many Different Transcription Factors Are Required for Hematopoiesis Differentiation of a specific blood cell typethe B lymphocyte As an example of hematopoiesis, we will discuss the development of one type of white blood cell, the B cell, in the adult bone marrow. To better understand their development, it is important to know what B cells do – they produce antibodies to fight infection Antibody genes are not encoded in our DNA in an assemble form, but rather need to be generated by DNA rearrangements that must take place during B cell development B Cell Development Hematopoietic stem cells give rise to a lymphoid progenitor cell, which is thought to be able to differentiate into both B cells and T cells as well as natural killer (NK) cells. This lymphoid progenitor can migrate to the thymus and initiate T cell development or remain in the bone marrow to initiate B cell development. Bone marrow B cell development can be subdivided into various stages: pro-B, pre-B, immature B and mature B cells. B Cell Development is Dependent on Several Factors A.) The presence of bone marrow stromal cells (a kind of fibroblast) providing cellmediated contacts and secreting the cytokine IL-7. B.) The rearrangement of the immunoglobulin (antibody) genes. In the common lymphoid progenitor both the immunoglobulin heavy and light chains are in their germ-line configuration (the unrearranged state) and must be rearranged (so they can give rise to an in-frame antibody protein) for B cell development to progress. C.) Expression of particular cytokine receptors and transcription factors by the developing B cell Bone Marrow Stromal Cell-Cell Contacts Bone marrow B lymphoid progenitors adhering to bone marrow stromal cells in culture. Bone marrow stromal cells provide at least two important contacts to B cell progenitors: an adhesive interaction through the integrin VLA-4 binding to VCAM-1 and a signaling one through surface bound SCF interacting with the Kit receptor on the lymphoid progenitor. Bone Marrow Stromal Cell Derived IL-7 IL-7 secreted by bone marrow stromal cells binds to the IL-7 receptor expressed on pro- and pre-B cells and stimulates the survival and proliferation of the B cells. Rearrangement of the Immunoglobulin Genes B cells exist to produce antibodies (immunoglobulins) Thus, B cell progenitors in the bone marrow will only survive and proliferate if they are able to rearrange the immunoglobulin genes to generate functional Immunoglobulins. How can a developing B cell tell if it has a functionallyrearranged immunoglobulin gene? The cells make a surface receptor out of the rearranged immunoglobulin and see if it signals! Surface Immunoglobulin Receptors on B Cell Progenitors Bone Marrow pro-B pre-B Heavy Chain Rearrangement Periphery immature B mature B activated B Light Chain Rearrangement Antibody Production In pre-B cells, the rearranged immunoglobulin heavy chain pairs with two other proteins l5 and V-preB, the surrogate light chains. This forms the pre-B cell receptor, which signals the cell to proliferate and further differentiate. Summary of B Cell Development Stromal Cell Dependence? Yes IL-7 Dependence? No Yes Yes Yes Yes No Yes No Yes No No No No