* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sedative-Hypnotic Drugs
Development of analogs of thalidomide wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
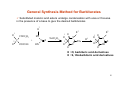
Sedative‐Hypnotic Drugs NEPHAR 305 Pharmaceutical Chemistry I 1 Assist.Prof.Dr. Banu Keşanlı Sedative-Hypnotic Drugs ¾ Chemical substances used to reduce tension and anxiety and induce calm (sedative effect) or to induce sleep and drowsiness (hypnotic effect). ¾ Most such drugs exert a quieting or calming effect at low doses and a sleep-inducing effect in larger doses. 2 Examples of Sedative-Hypnotic Drugs Barbiturates e.g. Methohexitone, Phenobarbital, Pentabarbital Thiopentone,Thiamylal Benzodiazepines e.g. Diazepam (Valium), Midazolam (Versed), Clonazepam (Klonopin) Nitrazepam (Ormodon) Flunitrazepam (Rohypnol) Glutethimide : Piperidinediones Meprobamate : Propanediol carbamates Alcohols : Ethanol, Chloral hydrate, Paraldehyde Buspirone : Azaspirodecanedione Zolpidem : Imidazopyridine Zaleplon : Pyrazolopyrimidine 3 Barbiturates – Derivatives of barbituric acid – Hypnotic/anxiolytic effect discovered in the early 20th century (Veronal®, 1903) – Until the 60s the largest group of hypnotics (more hypnotic than anxiolytic) – Act by both enhancing GABA responses and mimicking GABA (open Cl-channels in the absence of GABA) => increased inhibition of the CNS (also block glutamate receptors) – High risk of dependence (severe withdrawal symptoms) – Strong depressant activity on the CNS => anesthesia – At higher doses respiratory and cardiovascular depression => very little use today as hypnotics (only for epilepsy and anesthesia) 4 Barbiturates Different barbiturates vary mostly in their duration of action • Phenobarbital – Long-acting: used for anticonvulsive therapy 5-ethyl-5-phenylbarbituric acid • Thiopental – Very short acting (very lipophilic => redistributed from the brain into the fat tissue) ⇒CNS concentration falls below effective levels: used for i.v. anesthesia 5 General Synthesis Method for Barbiturates 9 Substituted malonic acid esters undergo condensation with urea or thiourea in the presence of a base to give the desired barbiturates 3 R 1 R 2 R COOC2H5 HN + C COOC2H5 1 C X H2N NaOC2H5 R R3 O 1 N XNa 2 R N H+ R R3 O N X 2 R NH O O X: O Barbitürik asit türevi X : O, barbituric acid derivatives X: S acid Tiyobarbitürik asit türevi X : S, thiobarbituric derivatives 6 Structure-Activity Relationship of Barbiturates The keto and enol tautomeric forms of barbituric acid with the sites of substitution in the hypnotically active barbiturates identified as 1, 2, and 5. Substitution at carbon 5 * Hypnotic activity is introduced 9 Branched chain --> greater hypnotic activity than straight chain 9 Phenol group (like phenobarbital) --> Greater anticonvulsant activity 9 Presence of hydrophilic groups such as –OH, -SH, -NH2, -COOH, -SO2 decreases lipophilicity so decreases activity Secobarbital phenobarbital 7 Structure-Activity Relationship of Barbiturates Carbon 2 * No substitution (oxygen) - oxybarbiturates (ex. pentobarbital and secobarbital) * Substitution with sulfur - thiobarbiturates (ex. thiopental and thiamylal) * Sulfuration at carbon 2 increases lipid solubility 9 Greater hypnotic potency 9 More rapid onset, but shorter duration of action e.g. thiopental has faster onset and shorter duration than pentobarbital Nitrogen 1 * Addition of a methyl group to nitrogen (e.g. methohexital) 9 Short duration of action 8 Hypnotics / Sedatives: Benzodiazepines ¾ Benzodiazepines have sedative, hypnotic, anti-anxiety, anticonvulsant, and muscle relaxant properties. ¾ They are the most widely used anxiolytic drugs used to treat anxiety, insomnia ,and a range of other conditions. ¾ Benzodiazepines have largely replaced barbiturates and meprobamate in the treatment of anxiety, because they are safer (less side effects and dependence) and more effective ¾ At low doses are useful sedatives and high doses produce a hypnotic effect ¾ Absorption and distribution: The benzodiazepines are lipophilic, and they are rapidly and completely absorbed after oral administration and distribute throughout the body. ¾ The benzodiazepines are excreted in the urine as glucuronides or oxidized metabolites. 9 Benzodiazepines 9 Derivatives of Benzodiazepine 9 Valium (diazepam) in 1962 9 Characteristic seven-membered ring fused to aromatic ring 9 Used to treat anxieties of all kinds (phobias, preoperative anxiety, myocardial infarction (prevent cardiac stress due to anxiety…) Mechanism of Action of Benzodiazepines Benzodiazepines work by enhancing the effect of the neurotransmitter gamma-aminobutyric acid (GABA) - which is responsible for reducing the activity of neurons that cause stress and anxiety. 10 The Core Structure of Benzodiazepines ¾ Heterocyclic ring system which is a fusion between benzene and diazepine ring system. ¾ This heterocycle has two nitrogen atoms, five carbon atoms and maximum possible number of cumulative double bonds. ¾ “Benzo” prefix benzene ring fused onto the diazepin ring ¾ "R" labels denote common locations of side chains, which give different benzodiazepines their unique properties. Left: The 1,4-benzodiazepine ring system. Right: 5-phenyl-1H-benzo[e][1,4]diazepin-2(3H)-one forms the skeleton of many of the most common benzodiazepine pharmaceuticals, such as diazepam (7-chloro-1-methyl substituted). 11 Diazepam (valium) 7- Chloro-1,3-dihydro-1-methyl-5-phenyl-1,4benzodiazepin-2-one 12 What are the different types of benzodiazepines? There are many different benzodiazepines and they all have differences in potency, speed at which they are metabolized, and "half-life" (time required for the quantity of the drug in the bloodstream to decrease to half its value), and therapeutic use. ¾ Different benzodiazepines vary mostly in their duration of action ¾ A substituent in the 7 position, such as a halogen or a nitro group, is required for sedative-hypnotic activity Clonazepam Diazepam Lorazepam Flunitrazepam Clonazepam Naming: 5-(2-chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzodiazepin-2-one 13 14 General Synthesis Method for Benzodiazepines Reaction of 2-aminobenzophenone derivatives and chloroacetyl chloride, followed by reaction with ammonia gives 1,3-dihydro-2H-1,4-benzodiazepin-2-on derivatives. Alkylation will then gives 1-substituted-1,3-dihydro-2H-1,4-benzodiazepin-2-on derivatives NH3 R1 C O R4 NH C CH Cl R4 ClCOCHCl O R1 R5 N R1 NH3 R5 R2 R2 X C O O R4 N H R5 H N R1 O R4 Clonazepam Klonazepam Nitrazepam Nitrazepam H N Lorazepam Nordazepam Lorazepam R5 Nordazepam Oksazepam Oxazepam Diazepam Flunitrazepam Diazepam Flurazepam Flunitrazepam Halazepam Flurazepam Nimetazepam Pinazepam Pinazepam Pirazepam Prazepam Temezepam Lormetazepam Lormetazepam 15 Diazepam (Valium Synthesis) H N CH3 Ac2 O (1) Cl 4-Chloro-Nmethylaniline NaOH, H2 O (3) O O N CH3 O PhCCl, AlCl3 (2) Cl Cl CH3 N H O Cl Cl Cl (4) CH3 O CH3 O N O O Cl (B) Cl NH3 (5) (A) CH3 O N CH3 O N Cl N O NH2 (6) Cl N Diazepam 16 Synthesis of Diazepam 17 Metabolism of Diazepam N-desmethyldiazepam conjugates conjugates 18 Metabolism of Clonazepam H N Redüksiyon reduction O reduction Redüksiyon OH O 2N H N N Cl 7-Asetamidoklonazepam 3-Hidroksiklonazep am 3-Hydroxyclonazepam Glükuronit ve& sülfat Glucuronic sulfate konjügatları conjugation Hidroksilasyon hydroxylation H N O acetylation Asetilasyon OH H 2N N Cl 7-acetaminoclonazepam hydroxylation Hidroksilasyon Hidroksilasyon hydroxylation O CH 3CONH 7-aminoclonazepam Clonazepam H N acetylation Asetilasyon 7-Aminoklonazepam Klonazepam H N O N Cl H 2N N Cl O 2N H N O N Cl 3-Hidroksi-7-aminoklonazepam 3-Hydroxy-7-aminoclonazepam Glükuronit ve sülfat Glucuronic & sulfate konjügatları conjugation O OH CH 3CONH N Cl 3-Hidroksi-7-asetamidoklonazepam 3-Hydroxy-7-acetaminoclonazepam Glükuronit&ve sülfat Glucuronic sulfate konjügatları conjugation 19 Structure Activity Relationship of Benzodiazepines ¾ ¾ ¾ In ring A an electron withdrawing group such as Cl, Br, NO2, or CN at position 7 increases activity A small group like methyl group attached to the nitrogen atom in position 1 in ring B has optimal activity. However, substituents at position 1 that are metabolically related are still clinically useful e.g. Flurazepam. Replacement of the carbonyl function with two hydrogens in position 2 gives medazepam, less effective than diazepam. 20 Structure Activity Relationship of Benzodiazepines ¾ Introduction of a carbonyl function in the 3 position increases the duration of action and also favours formation of water soluble salts. ¾ A phenyl substituent at 5 position increases activity. If this phenyl group has electronegative substituents such as Cl or F at the ortho and di ortho positions activity is improved. ¾ Saturation of the 4,5- double bond reduces potency, as does a shift of the unsaturation into the 3,4-position. 21 Chloral Hydrate Chloral hydrate, (trichloroacetaldehyde monohydrate) a sedative, is used in the short-term treatment of insomnia and to relieve anxiety and induce sleep before surgery. CH3 CH2 OH Cl2 Cl3C CH2 OH oxidation Oks. OH Cl3C CHO H2O Cl3C CH OH 2,2,2-trichloroethane-1,1-diol Metabolism: Chloral hydrate is converted to the active metabolite, trichloroethanol, in the body. OH Cl3C Cl3C CH OH CHO oxidation Redüksiyon reduction Oksidasyon Cl3C CH 2 OH Trikloroetanol trichloroethanol Cl3C COOH Trikloroasetik asit trichloroacetic acid Konjügasyon conjugation COOH Cl3C CH 2 O O 22 HO OH OH Buspirone Buspirone is useful in the treatment of generalized anxiety disorder and has an efficacy comparable to that of the benzodiazepines. 8-[4-(4-pyrimidin-2-ylpiperazin-1-yl)butyl]-8-azaspiro[4.5]decane-7,9-dione Synthesis: begins with N-alkylation 1-(2-pyrimidyl)piperazine with 4-chlorobutyronitrile followed by hydrogenation nitrile over Raney nickel catalyst. The primary amine product of the previous step is reacted with the spirocyclic acid anhydride in order to yield buspirone. 23 Meprobamate Meprobamate (Miltown, Equanil, Meprospan) is a carbamate derivative which is used as an sedative drug. 2-methyl-2-propyl-1,3-propandiol dicarbamate Synthesis: by the reaction of 2-methylvaleraldehyde with two molecules of formaldehyde and the subsequent transformation of the resulting 2-methyl-2-propylpropan-1,3-diol into the dicarbamate via successive reactions with phosgene and ammonia 24 Other Nonbenzodiazepine Sedatives The hypnotic zolpidem (Ambien, Stilnox) is not a benzodiazepine in structure, but it acts on a subset of the benzodiazepine receptor family Zaleplon (Sonata, Starnoc and Andante) is very similar to zolpidem in its hypnotic actions, but it causes fewer residual effects functions compared to zolpidem or the benzodiazepines. Eszopiclone (Lunesta) is an oral nonbenzodiazepine hypnotic and is also used for treating insomnia. Ethanol has anxiolytic and sedative effects, but its toxic potential outweighs its benefits. CH3CH2OH 25