* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Advanced in Antibody Design
Drug discovery wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Point mutation wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Ligand binding assay wikipedia , lookup
Immunoprecipitation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Drug design wikipedia , lookup
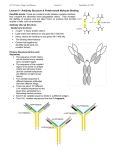
Advanced in Antibody Design [Annu. Rev. Biomed. Eng. 17 pp. 191-216 (2015)] Optimization of therapeutic antibodies binding affinity, specificity, folding stability, solubility, pharmacokinetics effector functions, bispecific antibodies, antibody-drug conjugates etc. Designing antibodies binding loops, scaffolds, domain interfaces, constant regions, post-translational and chemical modifications, bispecific architectures, antibody fragments 1. Introduction Antibody: humoral immunity, high affinity and specificity, adaptor molecules for effector functions IgA, IgD, IgE, IgG, IgM (heavy chains) IgG: 4 subclasses - abundance and effector functions / location and number of S-S bonds two light chains + two heavy chains Fab (antigen-binding fragment): CDR (complementarity-determining regions) Fc (crystallizable fragment): CH2 and CH3 (effector function) Molecular architecture of an immunoglobulin G1 (IgG1) antibody. An IgG consists of two heavy chains (blue) and two light chains ( pink). (Left) The crystal structure of an antigen-binding fragment (Fab; Protein Data Bank identification number, 3NZ8). The Fab is composed of variable heavy (VH) and light (VL) domains as well as two constant domains (CH1 and CL). Each variable domain displays three binding loops (complementarity-determining regions, CDRs), which mediate antigen recognition. The CDRs in the VH domain are denoted as H1, H2, and H3 (blue); the CDRs in the VL domain are denoted as L1, L2, and L3 ( pink). (Right) The crystallizable fragment (Fc; Protein Data Bank identification number, 1E4K) contains two constant domains (CH2 and CH3) as well as glycans in the CH2 domain ( green). The Fc fragment mediates antibody effector function. Polyclonal Ab vs. Monoclonal Ab (mAb) mAbs for therapeutics mAbs: excellent pharmacokinetics (long circulation time), low cytotoxicity & immunogenicity (human or humanized Ab), high stability & solubility, simplicity in production (cloning, expression, purification) Challenges of mAbs for therapeutic applications: immunization (antigen presentation) phage and yeast surface display (Ab fragments, not for intact Ab) solubility and viscosity at the high conc. bispecific antibody preparation antibody-drug conjugates antibodies with specific types and levels of effector functions simultaneous optimization of multiple antibody properties 2. Overview of Approaches for Designing Antibodies Key attributes of Ab (Fig. 2) Design efforts: redesigning or optimizing existing Abs rather than on de novo design of new Abs Key attributes of antibodies that must be collectively optimized to generate effective immunoglobulins for different applications. A key challenge is that optimizing one property can lead to deleterious impacts on others. 3. Antibody Binding Affinity and Specificity Abs: target recognition with high affinity and specificity CDR-mediated target binding CDR designing from motif-grafting to directed evolution 3-1. De Novo Design CDR Sequence prediction of Abs for Ag binding in high affinity and specificity OptCDR (Optimal Complementarity Determining Regions): Computational approach cf.) experimental approach 3-2. Design by Mimicking Natural Protein Interactions (CDR3) Ex) Ab for Prion protein (PrP): natural interaction between PrPC and PrPSc Ex) Amyloidogenic peptides such as Alzheimer’s Ab42 3-3. Semi-rational Design Combined with Directed Evolution Methods Designing some CDR residues while randomizing others ---- then, in vitro display methods (RGD in Ab for integrin binding) Abs to recognize post-translational modifications such as phosphorylation introduction of common phosphate-binding motif into CDR of Abs (Fig. 3) phosphoserine, phosphothreonine, phosphotyrosine Nature-inspired design and evolution of phospho-specific antibodies. This approach uses natural anion-binding motifs within the complementarity-determining regions (CDRs) of antibodies to generate libraries for identifying antibodies specific for phosphoserine, phosphotyrosine, and phosphothreonine (20). The first round of randomization and selection yields antibodies with anion-binding motifs specific for each type of modification, and the second round identifies antibodies with heavy chain CDR3 (HCDR3) and light chain CDR3 (LCDR3) loops that are specific for different phosphopeptides. 3-4 Antibody Redesign and Optimization - Immunization (Abs with low affinity) and Directed Evolution (unrealistic large library) - Redesign and Optimization Amino acid residue replacements (non-covalent interactions) electrostatic interactions > van der Waals interactions cf.) crystal structures and computational docking methods 4. Antibody Conformational (Folding) Stability - to maintain long-term activity - during CDR grafting - how to stabilize Abs a) knowledge-based: stabilizing mutations or scaffolds (general) b) statistical: consensus approaches (항체대상) c) structure-based methods: computational approaches Ex) bispecific Abs: fusion between IgG and scFv (single-chain variable fragment) Fig. 4 (Four stabilizing mutations: S16E, V55G, P101D for VH/ S46L for VL) - introduction of intramolecular or interdomain S-S bonds - non-cysteine mutations at VH-VL interface Mutations identified using structure-based and related design methods that enhance the conformational (folding) stability of antibody fragments. Four mutations were introduced into the variable domains of a poorly stable antibody fragment (red ) that generated a more stable one ( green) (44). The crystal structures were obtained from the Protein Data Bank: (top) 3HC0 and (bottom) 3HC4. Abbreviations: λem, average (center of mass) fluorescence emission wavelength. 5. Antibody Colloidal Stability (Solubility) - solvent exposed residues in native folded structure - Abs a) CDRs, b) frameworks of the variable and constant domains, c) glycans - increase Ab solubility without reducing its binding activity - Fig. 5 (Ab for glycoprotein LINGO-1) 6. Antibody Effector Function - ADCC (antibody-dependent cell-mediated cytotoxicity) and ADCP (… phagocytosis) Fc domain and Fcg on NK cells, macrophages, other immune cells - CDC (complement-dependent cytotoxicity) Fc and C1q (cellular and noncellular mechanisms of cytotoxicity) - two key approaches a) engineering the sequences of Fc and hinge regions b) modulating the amount and type of Fc glycosylation - Fc receptor binding site analysis (Fcg receptors and C1q) optimization of Fc domains for different therapeutic applications Ex) Fc sequences near Fcg and C1q binding sites (increase both ADCC and CDC effector fn) Ex) alter the amount and type of glycosylation Fc modifications of aglycosylated Abs composition of glycans Structure-guided design and selection of crystallizable fragment (Fc) mutations that increase complement-dependent cytotoxicity (CDC). (a) Residues in the heavy chain constant domain CH2 (Protein Data Bank identification number, 1E4K) that form the putative C1q binding center. (b) Mutations identified using structure-based methods that increase CDC (85). Evaluation of (c) CDC and (d) antibody-dependent cell-mediated cytotoxicity (ADCC) of wild-type and three mutants of an immunoglobulin G1 (IgG1) anti-CD20 antibody. 7. Antibody-drug conjugates - improving the cytotoxicity of Ab therapeutics - problems for the generation of safe and effective drug conjugates a) reduced bioactivity of conjugated drugs b) reduced binding affinity or specificity of modified antibodies c) premature release of the conjugated drug d) insufficient cellular internalization e) short circulation times and poor biodistribution - systematic optimization a) antibodies and drugs themselves b) site-specific modifications (Fab, Fc, Amino acids, CHO etc.) c) drug attachment chemistry d) crosslinkers between Ab and drug Ex) attaching drugs to engineered cysteines at different sites in the Fab and Fc domains maleimide-based conjugation light chain V205C: least solvent-accessible cysteine (high ADC) Fc S396C: most accessible cysteine (low ADC) - ratios of drugs to Ab, crosslinker between Ab and drug etc. Design and evaluation of the bioactivity of antibodies conjugated at different sites with a cytotoxic drug. (a) The sites mutated to cysteine are highlighted in the crystal structures of the antigen-binding fragment (Fab) and crystallizable fragment (Fc), and (b) these sites show a range of solvent accessibilities. (c) Evaluation of the clearance rates of antibodies injected into mice. Although the total antibody levels were similar for injections of each antibody–drug conjugate (ADC), the fraction of intact ADC was highest for the light chain (LC) variant V205C and lowest for the Fc S396C variant. (d ) Mice dosed equally with each ADC showed significant differences in survival, and these differences were consistent with the fraction of intact ADC. Abbreviation: HC, heavy chain. 8. Bispecific Antibodies - mAbs recognizing multiple targets - improvement of Ab activity a) improving effector function by targeting specific immune cells in addition to the therapeutic target b) enhancing Ab delivery to different organs in addition to the therapeutic target c) increasing specificity for pathogenic cells (targeting two cell-surface Ag) d) improving the robustness and persistence of therapeutic activity by blocking two different biological pathways - molecular architectures a) full-length Abs with two different heavy chains b) full-length Abs with additional variable domains c) Ab fragments lacking Fc domains (scFv, diabodies) - two different heavy chains a) correct heteropairing of the heavy chains b) correct pairing of the light chains with their corresponding heavy chains Quadroma Trimab: rat heavy chains (no Protein A binding) knobs-into-holes CrossMAb: CH1/CL crossover approach (for correct light-chain pairing) Dual-variable domains IgG-scFv Molecular architectures of bispecific monoclonal antibodies (mAbs). Two mAbs are recombined into different bispecific architectures. A quadroma Triomab (Trion Pharma, Munich, Germany) comprises one heavy chain–light chain pair of a rat immunoglobulin G2 (IgG2) and one heavy chain–light chain pair of a murine IgG2 antibody (128). The knobs-into-holes architecture consists of an opposing cavity and protrusion in the heavy chain constant CH3 domains to enforce the heteropairing of heavy chains (127). The CrossMAb (Roche, Basel, Switzerland) architecture involves swapping the light chain constant CL and the heavy chain constant CH1 domains onto opposite chains to enforce correct light-chain pairing, and also uses knobs-into-holes mutations to enforce correct heavy-chain pairing (140). Dual-variable-domain antibodies have the variable domains from one antibody added to the N terminus of the heavy and light chains of the other antibody (142). IgG–scFv (single-chain variable fragment) bispecific antibodies contain the variable domains of one antibody—which are reformatted as an scFv— fused to the terminus of the heavy or light chains of the second antibody (132). 9. Future Directions (1) De novo design of Abs structure-based, computational analysis (2) simultaneous optimization of multiple attributes of Abs CDR design, folding stability, solubility bispecific Abs with non-conventional architectures (3) optimizing Ab effector function (4) designing ADCs (Ab-drug conjugates) - producing Abs with properties that are uncommon or absent in conventional Abs - complex, non-conventional antibody formats different architectures, valancies, orientations and accessibilities of the variable domains, stabilities relative to conventional Abs