* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Bioinorganic_chemistry
Living things in culture wikipedia , lookup
Protein purification wikipedia , lookup
Photosynthesis wikipedia , lookup
Chemical biology wikipedia , lookup
Protein–protein interaction wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein adsorption wikipedia , lookup
Biochemistry wikipedia , lookup
Organisms at high altitude wikipedia , lookup
High-altitude adaptation in humans wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
BIOINORGANIC
CHEMISTRY
Presentation By:
Sitanshu Kumar
Introduction
Bioinorganic chemistry is concerned with the roles of
inorganic elements in biological processes.
Metal ions can have structural roles, catalytic roles, or both.
Metals that have catalytic roles will be present at the active site of the
biomolecule which will likely be a metalloprotein (a metalloenzyme).
The reactivity of a metalloprotein is defined by the nature of the metal,
particularly its electronic structure and oxidation state.
This, in turn, is determined by its coordination environment (ligand donor
atoms) and molecular geometry, which is provided by the architecture of
the protein surrounding the metal.
Biologically Important Elements
• 99% of human body is comprised of 11 elements:
• Bulk biological elements: H, C, N, O, P, S, Cl (as PO43-, SO42-, Cl-)
• Bulk metal ion nutrients: Na, Mg, K, Ca
• Essential elements for a wide range of bacteria/plants/animals
• Transition metals: V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Mo
• Non-Metals: (B), F, (Si), Se I, F.
Some Other Facts
• Mammals are believed to use only 25 of the known elements.
•
Eleven non-transition elements that make up 99.9% of the human
body (O, C, H, N, Ca, P, S, K, Cl, Na, Mg),
• Three transition metals, Fe, Zn and Cu are needed in significant
amounts.
• “Trace quantities” of many other transition elements are required to
maintain proper physical functioning.
• Other elements in the human body (e.g. Rb, Zr, Sr, Al, Pb, Ba) are not
essential but incorporated inadvertently because they share chemical
and physical properties with essential elements.
• Other elements are added to the list of elements thought to be essential
as our knowledge of the chemistry of living systems increases.
Symptoms of Elemental Deficiency
in Humans
Ca
Mg
Fe
Zn
Cu
Mn
Mo
Co
Ni
Cr
Si
F
I
Se
As
Retarded skeletal growth
Muscle cramps
Anemia, immune disorders
Stunted growth, skin damage, retarded maturation
Liver disorders, secondary anemia
Infertility, impaired skeletal growth
Retarded cellular growth
Pernicious anemia
Depressed growth, dermatitis
Diabetes symptoms
Skeletal growth disorders
Dental disorders
Thyroid disorders
Cardiac muscular weakness
Impaired growth (in animals)
Biological Roles of Metallic Elements
Structural
Skeletal roles via biomineralization
Ca2+, Mg2+, P, O, C, Si, S, F as anions, e.g. PO43, CO32.
Charge neutralization.
Mg2+, Ca2+ to offset charge on DNA - phosphate anions
Charge carriers: Na+, K+, Ca2+
Transmembrane concentration gradients ("ion-pumps and channels")
Trigger mechanisms in muscle contraction (Ca). Electrical impulses in nerves (Na, K)
Heart rhythm (K).
Hydrolytic Catalysts: Zn2+ , Mg2+
Lewis acid/Lewis base Catalytic roles. Small labile metals.
Redox Catalysts: Fe(II)/Fe(III)/Fe(IV), Cu(I)/Cu(II), Mn(II)/Mn(III)/(Mn(IV),
Mo(IV)/Mo(V)/Mo(VI), Co(I)/Co(II)/Co(III)
Transition metals with multiple oxidation states facilitate electron transfer - energy transfer. Biological
ligands can stabilize metals in unusual oxidation states and fine tune redox potentials.
Activators of small molecules.
Transport and storage of O2 (Fe, Cu)
Fixation of nitrogen (Mo, Fe, V)
Reduction of CO2 (Ni, Fe)
Organometallic Transformations.
Cobalamins, B12 coenzymes (Co), Aconitase (Fe-S)
Transition Metals in Biomolecules
Iron.
Most abundant metal in biology, used by all plants and animals including bacteria. Some roles
duplicated by other metals, while others are unique to Fe. Iron use has survived the evolution of
the O2 atmosphere on earth and the instability of Fe(II) with respect to oxidation to Fe(III).
Zinc.
Relatively abundant metal. Major concentration in metallothionein (which also serves as a
reservoir for other metals, e.g. Cd, Cu, Hg). Many well characterized Zn proteins, including redox
proteins, hydrolases and nucleic acid binding proteins.
Copper
Often participatse together with Fe in proteins or has equivalent redox roles in same biological
reactions. Reversible O2 binding, O2 activation, electron transfer, O2- dismutation (SOD).
Cobalt.
Unique biological role in cobalamin (B12-coenzymes) isomerization reactions.
Manganese
Critical role in photosynthetic reaction centers, and SOD enzymes.
Molybdenum
Central role in nitrogenase enzymes catalyzing N2 NH3, NO3 NH3
Chromium, Vanadium and Nickel
Small quantities, uncertain biological roles. Sugar metabolism (Cr);
Ni only in plants and bacteria (role in CH4 production) and SOD enzymes.
Biochemical Classification of
Metallobiomolecules
Transport and storage proteins :
O2 binding/transport:
Transferrin (Fe) , Ferritin (Fe), Metallothionein (Zn)
Myoglobin (Fe), Hemoglobin (Fe),Hemerythrin (Fe)
Hemocyanin (Cu)
Enzymes (catalysts)
Hydrolases:
Carbonic anhydrase (Zn), Carboxypeptidase (Zn)
Oxido-Reductases:
Alcohol dehydrogenase (Zn), Superoxide
dismutase (Cu, Zn,Mn, Ni), Catalase, Peroxidase (Fe),
Nitrogenase (Fe, Mo), Cytochrome oxidase (Fe, Cu),
Hydrogenase (Fe, Ni)
Isomerases:
B12 coenzymes (Co), Aconitase (Fe-S)
Oxygenases:
Cytochrome P450 (Fe), Nitric Oxide Synthases (Fe)
Electron carriers:
Cytochromes (Fe)
Electron transferases
Iron-sulfur (Fe)
Blue copper proteins (Cu)
Non Proteins
Transport Agents:
Siderophores (Fe)
Porphyrins and Related Complexes in
Bioinorganic Molecules
• A porphyrin ring has a square planar geometry with a
“pocket” in the center.
• A metalloporphyrin complex can result by
incorporating a metal atom into the pocket Axial sites
are available for other ligands.
• Structure, specificity, and reactivity are changed by
differing the side chains, metal ions, and surrounding
species.
Haemoglobin and Myoglobin
• Oxygen transfer and storage agents in the blood and muscle
tissue.
• Hemoglobin transports oxygen (O2) from the lungs/gills to tissues
and muscles.
• Myoglobin stores oxygen (O2) in the muscles and tissues.
Oxygen commonly transfers from the hemoglobin to the myoglobin
for later use.
Haemoglobin
• Made up of four globin protein subunits ( and
).
• Each protein partially encloses a heme group.
• Each heme group is in a porphyrin pocket.
• One axial position of the iron is bound to an
imidazole nitrogen from the protein.
• One axial position is available/vacant or has H2O
bound to it.
• Dissolved O2 can bind reversibly to this axial
position.
Haemoglobin (Structure)
Oxygen Addition to
Haemoglobin
Cooperativity
• Cooperativity:
The function of hemoglobin is to bind O2 at high oxygen
pressure and carry it through the blood to needed areas
(and myoglobin for storage).
Hb + 4O2 Hb(O2)4
Hb(O2)4 + 4Mb 4Mb(O2) + Hb
• As one iron binds an oxygen molecule in Hb, the
molecular shape changes to make binding of
additional oxygen molecules easier. In a similar
fashion, initial removal of oxygen triggers the
release of the remaining oxygens.
Bohr Effect & Comparision
• At low partial pressures
of O2, Mb has a much
greater affinity for O2.
K Mb
[Mb(O 2 )]
[Mb][O 2 ]
K Hb
[Hb (O 2 ) 4 ]
[Hb ][O 2 ]2.8
• The Bohr effect.
• Increased acidity favours
the release of O2 from
Hb(O2)4.
Structures
Myoglobin
Haemoglobin
Chlorophyll & Haemoglobin
Chlorophyll
Haemoglobin
Metalloenzymes
Zinc(II) in the active centre of
carboxipeptidase-A
The active centre of the alcohol
dehydrogenase
The supposed reaction mechanism of
dinitrogenase
Supposed structure of Fe-S-Mo cofactor of
nitrogenase
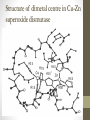
Structure of dimetal centre in Cu-Zn
superoxide dismutase