* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hydroxyl Compounds
Survey
Document related concepts
Transcript
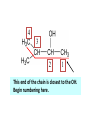
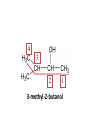
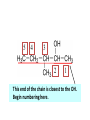
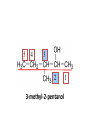
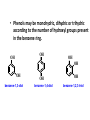
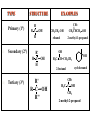
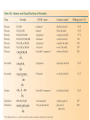
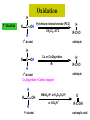
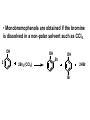
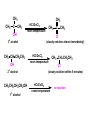
ORGANIC CHEMISTRY CHM 207 CHAPTER 6: HYDROXYL COMPOUNDS (ALCOHOLS AND PHENOL) NOR AKMALAZURA JANI SUBTOPICS • Nomenclature of alcohols, phenols. • Classification of alcohols. • Physical properties of alcohols: - Physical state - Boiling points - Solubility of alcohols in water • Acidity of alcohols and phenols • Reactions of alcohols: - Reaction with sodium - Oxidation - Esterification - Halogenation and haloform reactions - Dehydration - Formation of ether (Williamson ether synthesis) • Reactions of phenols: - Reaction with sodium - Esterification - Halogenation of the ring - Nitration of the ring • Tests to distinguish classes of alcohols: i) Lucas test ii) Oxidation • Haloform test to identify methyl alcohol group - Iodoform - Bromoform • Uses of alcohols and phenols. ALCOHOLS • Alcohols: Organic compounds containing hydroxyl (-OH) functional groups. R OH Phenols: Compounds with hydroxyl group bonded directly to an aromatic (benzene) ring. OH NOMENCLATURE OF ALCOHOLS IUPAC RULES 1. 2. 3. 4. Select the longest continuous chain of carbon atoms containing the hydroxyl group. Number the carbon atoms in this chain so that the one bonded to the –OH group has the lowest possible number. Form the parent alcohol name by replacing the final –e of the corresponding alkane name by –ol. When isomers are possible, locate the position of the –OH by placing the number (hyphenated) of the carbon atom to which the –OH is bonded immediately before the parent alcohol name. Name each alkyl branch chain (or other group) and designate its position by number. This is the longest continuous chain that contains an hydroxyl group. Select this chain as the parent compound. 4 3 2 1 This end of the chain is closest to the OH. Begin numbering here. 4 3 2 1 3-methyl-2-butanol This is the longest continuous chain that contains an hydroxyl group. Select this chain as the parent compound. 5 4 3 2 1 This end of the chain is closest to the OH. Begin numbering here. 5 4 3 2 3-methyl-2-pentanol 1 NOMENCLATURE OF CYCLIC ALCOHOLS • Using the prefix cyclo• The hydroxyl group is assumed to be on C1. H OH 6 1 5 2 43 Br IUPAC name: HO CH2CH3 1 H trans-2-bromocyclohexanol new IUPAC name: trans-2-bromocyclohexan-1-ol 3 2 1-ethylcyclopropanol 1-ethylcyclopropan-1-ol NOMENCLATURE OF ALCOHOLS CONTAINING TWO DIFFERENT FUNCTIONAL GROUPS • Alcohol containing double and triple bonds: - use the –ol suffix after the alkene or alkyne name. • The alcohol functional group takes precedence over double and triple bonds, so the chain is numbered in order to give the lowest possible number to the carbon atom bonded to the hydroxyl group. • The position of the –OH group is given by putting its number before the –ol suffix. • Numbers for the multiple bonds were once given early in the name. EXAMPLE 5 4 CH2 CH 3 CH2 2 CH 1 CH3 OH 1) Longest carbon chain that contains –OH group - 5 carbon 2) Position of –OH group - Carbon-2 3) Position of C=C - Carbon-4 COMPLETE NAME = 4-penten-2-ol • Some consideration: - OH functional group is named as a hydroxy substituent when it appears on a structure with a higher priority functional group such as acids, esters, aldehydes and ketones. - Examples: OH 4 CH3 3 CH O 2 CH2 1 C OH 3-hydroxybutanoic acid 4 3 2 5 1 6 OH O 2-hydroxycyclohexanone MAIN GROUPS decreasing priority Acids Esters Aldehydes Ketones Alcohols Amines Alkenes Alkynes Alkanes Ethers Halides NOMENCLATURE OF DIOLS • Alcohols with two –OH groups are called diols or glycols. • Naming of diols is like other alcohols except that the suffix diol is used and two numbers are needed to tell where the two hydroxyl groups are located. 5 OH 3 CH3 IUPAC name 2 CH 1 CH2 propane-1,2-diol 1 4 OH OH 3 2 OH trans-cyclopentane-1,2-diol NOMENCLATURE OF PHENOLS • The terms ortho (1,2-disubstituted), meta (1,3-disubstituted) and para (1,4-disubstituted) are often used in the common names. OH O2N OH CH3CH2 Br IUPAC name: 2-bromophenol common name: ortho-bromophenol OH 3-nitrophenol meta-nitrophenol 4-ethylphenol para-ethylphenol • Phenols may be monohydric, dihydric or trihydric according to the number of hydroxyl groups present in the benzene ring. OH OH OH OH OH benzene-1,3-diol OH benzene-1,4-diol OH benzene-1,2,3-triol CLASSIFICATION • According to the type of carbinol carbon atom (C bonded to the –OH group). C OH Classes: i) Primary alcohol - -OH group attached to a primary carbon atom ii) Secondary alcohol - -OH group attached to a secondary carbon atom iii) Tertiary alcohol - -OH group attached to a tertiary carbon atom TYPE i) ii) Primary (1°) Secondary (2°) STRUCTURE H R C H EXAMPLES CH3 OH R' R C OH H CH3CH2-OH CH3CHCH2 OH ethanol 2-methyl-1-propanol OH 2-butanol iii) Tertiary (3°) R' R C OH R'' OH H3C CH CH2CH3 cyclohexanol CH3 H3C C OH CH3 2-methyl-2-propanol Polyhydroxy Alcohols • Alcohols that contain more than one OH group attached to different carbons are called polyhydroxy alcohols. • Monohydroxy: one OH group per molecule. • Dihydroxy: two OH groups per molecule. • Trihydroxy: three OH groups per molecule. PHYSICAL PROPERTIES • PHYSICAL STATES OF ALCOHOLS - simple aliphatic alcohols and lower aromatic alcohols (such as phenylmethanol, C6H5CH2OH) → liquids at room temperature. - highly branched alcohols and alcohols with twelve or more carbon atoms → solids. • BOILING POINTS - The boiling points of alcohols are higher than those of alkanes and chloroalkanes of similar relative molecular mass. - For example: C2H5OH Relative molecular mass: Boiling point: 46 CH3CH2CH3 44 78°C CH3Cl 50.5 -42°C -24°C - Reason: intermolecular hydrogen bonds exist between the –OH groups in the alcohol molecules. Ar δδ+ R δδ+ Ar O H R O H OδOδH hydrogen bonding H hydrogen bonding - Branched chain alcohols boils at a lower temperature (more volatile) than the straight chain alcohols with the same number of carbon atoms. • SOLUBILITY OF ALCOHOLS IN WATER i) alcohols with short carbon chains (such as methanol, ethanol, and propanol) dissolve in water. - when alcohols dissolve in water, hydrogen bonds are formed between the –OH group of the alcohol molecule and the –OH group of the water molecule. ii) the solubility of alcohols in water decreases sharply with the increasing length of the carbon chain. Higher alcohols are insoluble in water. - alcohol contains a polar end (-OH group) called ‘hydrophilic’ and a nonpolar end (the alkyl group) called ‘hydrophobic’. - the water solubility decreases as the alkyl group becomes larger. iii) alcohols with more than one hydroxyl group (polyhydroxy alcohols) are more soluble than monohydroxy alcohols with the same number of carbon atoms. This is because they can form more hydrogen bonds with water molecule. iv) branched hydrocarbon increases the solubility of alcohol in water. - reason: branched hydrocarbon cause the hydrophobic region becomes compact. * Phenol is unusually soluble (9.3%) because of its compact shape and the particularly strong hydrogen bonds formed between phenolic – OH groups and water molecules. ACIDITY OF ALCOHOLS AND PHENOLS • Alcohol is weakly acidic. • In aqueous solution, alcohol will donated its proton to water molecule to give an alkoxide ion (R-O-). R-O- + H3O+ R-OH + H2O Ka = ~ 10-16 to 10-18 alkoxide ion Example CH3CH2-OH + H2O CH3CH2-O- + H3O+ The acid-dissociation constant, Ka, of an alcohol is defined by the equilibrium R-OH + H2O Ka Ka = [H3O+] [RO-] [ROH] R-O- + H3O+ pKa = - log (Ka) * More smaller the pKa value, the alcohol is more acidic Acidity OF PHENOLS • Phenol is a stronger acid than alcohols and water. R-OH + H2O alcohol OH R-O- + H3O+ Ka = ~ 10-16 to 10-18 alkoxide ion H2O phenol H 2 O + H2 O O- H3O+ Ka = 1.2 x 10-10 phenoxide ion HO- + H3O+ hydroxide ion Ka = 1.8 x 10-16 • Phenol is more acidic than alcohols by considering the resonance effect. i) The alkoxide ion (RO-) - the negative charge is confined to the oxygen and is not spread over the alkyl group. - this makes the RO- ion less stable and more susceptible to attack by positive ions such as H+ ions. ii) The phenoxide ion - one of the lone pairs of electrons on the oxygen atom is delocalised into the benzene ring. - the phenoxide ion is more stable than the alkoxide ion because the negative charge is not confined to the oxygen atom but delocalised into the benzene ring. - the phenoxide ion is resonance stabilised by the benzene ring and this decreases the tendency for the phenoxide ion to react with H3O+. O O O O EFFECTS OF Acidity • The acidity decreases as the substitution on the alkyl group increase. - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electron withdrawing atom helps to stabilize the alkoxide ion. - For example: 2-chloroethanol is more acidic than ethanol because the electron-withdrawing chlorine atom helps to stabilize the 2-chloroethoxide ion. - alcohol with more than one electron withdrawing atoms are more acidic. For example, 2,2,-dichloroethanol is more acidic than 2-chloroethanol. - Example of electron-withdrawing atom/groups: Halogen atoms and NO2. REACTIONS OF ALCOHOLS • • • • • • Reaction with sodium Oxidation Esterification Halogenation and haloform reactions Dehydration Formation of ether (Williamson ether synthesis) Reaction with sodium • Alcohols reacts with Na at room temperature to form salts (sodium alkoxides) and hydrogen. 2R-O-H + 2Na → 2R-O- Na+ + H2 For example: CH3CH2OH + Na → CH3CH2O-Na+ + 1/2H2 alcohol sodium ethoxide Reactivity of alcohols towards the reactions with sodium: CH3 > 1° > 2° > 3° Oxidation H 1° alcohol R C OH Pyridinium chlorochromate (PCC) H CH2Cl2, 25oC R-C=O H 1o alcohol aldehyde H R C OH Cu or Cr3O/pyridine H R-C=O H 1o alcohol Cr3O/pyridine = Collins reagent aldehyde H R C OH H 1o alcohol KMnO4/H+ or K2Cr2O7/H+ or CrO3/H+ O R-C-OH carboxylic acid Examples: 1° alcohol O CH3(CH2)4-CH2-OH PCC 1-hexanol CH3(CH2)4-CH2-OH 1-hexanol CH3(CH2)4-C-H hexanal KMnO4/H+ or K2Cr2O7/H+ or CrO3/H+ O CH3(CH2)4-C-OH hexanoic acid H + 2° alcohol R C OH + KMnO4/H or K2Cr2O7/H O or CrO3/H+ R' R-C-R' ketone 2o alcohol R" 3° alcohol R C OH KMnO4/H+ or K2Cr2O7/H+ no reaction + or CrO3/H R' 3o alcohol Example: OH CH3 CH CH2CH3 2-butanol + + KMnO4/H or K2Cr2O7/H or CrO3/H+ O CH3 C CH2CH3 2-butanone Esterification • Esterification: - the reaction between an alcohol and a carboxylic acid to form an ester and H2O. O R C O H H O carboxylic acid CH3CH2-O-H ethanol R' O CH3 C methanol O R' H2O O H+ O H ethanoic acid C OH benzoic acid C ester O CH3-O-H R alcohol EXAMPLES H+ = catalyst O H+ CH3 C OCH2CH3 ethyl ethanoate H+ O C OCH3 methyl benzoate H2O H2O Esterification also occurs when alcohols react with derivatives of carboxylic acids such as acid chlorides O O CH3-O-H CH3 C Cl CH3 C OCH3 methanol ethanoyl chloride methyl ethanoate HCl Halogenation and haloform reactions 1) Hydrogen halides (HBr or HCl or HI) R-OH + H-X → R-X + H2O Example: C2H5-OH + H-Br • • C2H5-Br + H2O H+ Reactivity of hydrogen halides decreases in order HI > HBr > HCl Reactivity of alcohols with hydrogen halides: 3° > 2° > 1° 2) Phosphorus trihalides, PX3 3R-OH + PX3 3R-X + H3PO3 (PX3 = PCl3 or PBr3 or PI3) Example: (CH3)2CHCH2-OH + PBr3 → (CH3)2CHCH2-Br isobutyl alcohol isobutyl bromide 3) Thionyl chloride (SOCl2) R-OH + SOCl2 → R-Cl + SO2 + HCl Example: CH3(CH2)5CH2-OH + SOCl2 → CH3(CH2)5CH2-Cl + SO2 + HCl 1-heptanol 1-chloroheptane Dehydration • Dehydration of alcohols will formed alkenes and the products will followed Saytzeff rules. R-CH2-CH2-OH conc. H2SO4 R-CH=CH2 + H2O Saytzeff rule: - A reaction that produces an alkene would favour the formation of an alkene that has the greatest number of substituents attached to the C=C group. + H CH3CH2-CH-CH3 OH 2-butanol CH3CH2-CH=CH2 + H2O + H 1-butene CH3CH=CH-CH3 + H2O 2-butene major product • Reactivity of alcohols towards dehydration: 3° > 2° > 1° • Reagents for dehydration: i) Concentrated H2SO4 CH3-CH2-OH conc. H2SO4 CH2=CH2 + H2O ii) With phosphoric (v) acid OH 85% H3PO4, 165-170oC iii) Vapour phase dehydration of alcohols CH3CH2OH Al2O3 heat CH2=CH2 + H2O H2O Formation of ether (Williamson ether synthesis) • Involves the SN2 attack of an alkoxide ion on an unhindered primary alkyl halides. • The alkoxide is made by adding Na, K or NaH to the alcohol. R-O- + R’-X → R-O-R’ + Xalkoxide (R’ must be primary) The alkyl halides (or tosylate) must be primary, so that a back-side attack is not hindered. If the alkyl halides is not primary, elimination usually occurs to form alkenes. EXAMPLES CH3CH2-OH + Na CH3CH2-O Na CH3CH2-O-CH3 CH3I NaI or CH3CH2-OH OH 1) Na 2) CH3I 1) Na CH3CH2-O-CH3 OCH2CH3 2) CH3CH2-OTs cyclohexanol ethoxycyclohexane NaI REACTIONS OF PHENOLS • • • • Reaction with sodium Esterification Halogenation of the ring Nitration of the ring REACTION WITH SODIUM OH Na - O Na+ 1/2 H2(g) sodium phenoxide REACTION WITH AQUEOUS SODIUM HYDROXIDE OH NaOH - O Na+ sodium phenoxide ROH + NaOH no reaction H2O ESTERIFICATION EXAMPLES O OH NaOH ONa H2O O CH3CCl NaCl OCCH3 NaOH sodium phenoxide O OH C OH O H+ OC phenyl benzoate H2O HALOGENATION • More reactive towards electrophilic substitution than benzene. • ortho-para director. OH OH X 3X2 X room temperature 3HX X EXAMPLES OH OH Br 3Br2 Br room temperature 3HBr Br 2,4,6-tribromophenol (white precipitate) OH OH Cl 3Cl2 Cl room temperature 3HCl Cl 2,4,6-trichlorophenol (white precipitate) • Monobromophenols are obtained if the bromine is dissolved in a non-polar solvent such as CCl4. OH 2 OH OH Br 2Br2 (CCl4) 2HBr Br NITRATION • Dilute nitric (v) acids reacts with phenol at room temperature to give a mixture of 2- and 4-nitrophenols. OH 2 OH OH 2HNO3 < 20oC NO2 2H2O NO2 2-nitrophenol 4-nitrophenol By using concentrated nitric (v) acid, the nitration of phenol yields 2,4,6-trinitrophenol (picric acid). Picric acid is a bright yellow crystalline solid. It is used in the dyeing industry and in manufacture of explosives. OH OH 3HNO3 O2 N NO2 NO2 2,4,6-trinitrophenol (picric acid) 3H2O TESTS TO DISTINGUISH CLASSES OF ALCOHOLS 1) Lucas Test - The alcohol is shaken with Lucas reagent (a solution of ZnCl2 in concentrated HCl). - Tertiary alcohol - Immediate cloudiness (due to the formation of alkyl chloride). - Secondary alcohol - Solution turns cloudy within about 5 minutes. - Primary alcohol - No cloudiness at room temperature. CH3 CH3 C CH3 OH CH3 HCl/ZnCl2 room temperature Cl o 3 alcohol CH3 CH CH2CH3 OH (cloudy solution almost immediately) HCl/ZnCl2 room temperature (cloudy solution within 5 minutes) HCl/ZnCl2 room temperature 1o alcohol CH3 CH CH2CH3 Cl 2o alcohol CH3CH2CH2CH2OH CH3 C CH3 no reaction 2) Oxidation of alcohols - only primary and secondary alcohols are oxidised by hot acidified KMnO4 or hot acidified K2Cr2O7 solution. - the alcohol is heated with KMnO4 or K2Cr2O7 in the presence of dilute H2SO4. - 1o or 2o alcohol: → the purple colour of KMnO4 solution disappears. → the colour of the K2Cr2O7 solution changes from orange to green. - 3o alcohol do not react with KMnO4 or K2Cr2O7. + Cr2O2-7 + 8H+ 3RCH2OH 1o alcohol (orange) (orange) 3RCOOH + 2Cr3+ + 7H2O (green) carboxylic acid R' R' 3 R CH OH + Cr2O2-7 + 8H+ 2o alcohol (green) aldehyde 3RCHO + Cr2O2-7 + 8H+ aldehyde + 2Cr3+ + 7H2O 3RCHO (orange) 3R C ketone 3+ + 2Cr + 7H2O O (green) HALOFORM TEST TO IDENTIFY METHYL ALCOHOL GROUP 1) Iodoform: • Ethanol and secondary alcohols containing the group methyl alcohol group which react with alkaline solutions of iodine to form triiodomethane (iodoform, CHI3). • Triiodomethane – a pale yellow solid with a characteristic smell. H CH3 C OH (methyl alcohol group) H CH3 C R + 4I2 + 6NaOH OH CHI3 (s) + RCOONa + 5NaI + 5H2O triiodomethane (iodoform) yellow precipitate where R = hydrogen, alkyl or aryl group • The iodoform test can distinguish ethanol from methanol H CH3 C O H + 4I2 + 6OH - CHI3 (s) + 5I + 5H2O iodoform OH H C O methanoate ethanol positive iodoform test H H C H + 4I2 + 6OH OH methanol no reaction negative iodoform test • The iodoform test can distinguish 2-propanol from 1-propanol CH3 CH3 C O CHI3 (s) + 5I- + 5H2O H + 4I2 + 6OH 2-propanol H H H H C C C H H OH 1-propanol C O ethanoate iodoform OH CH3 positive iodoform test H + 4I2 + 6OH no reaction negative iodoform test * TERTIARY ALCOHOLS DO NOT GIVE POSITIVE IODOFORM TEST 2) BROMOFORM H CH3 C R + 4Br2 + 6NaOH CHBr3 (s) + RCOONa + 5NaBr + 5H2O bromoform OH where R = hydrogen, alkyl or aryl group reagent sample iodoform USES OF ALCOHOLS • As solvents: - examples: the lower alcohols such as methanol, ethanol and propanol. - methanol is used as a solvent for varnish and paints. • As fuels: - biofuel (fuel derived from a biological source). - ethanol can be produced from sugars such as sucrose from sugar cane, through fermentation and distillation. It can be blended with petrol and used as fuel in motor vehicles. - methylated spirit is ethanol made undrinkable by the addition of a little methanol. It is used as a fuel in camping stoves. • In alcoholic drinks: - ethanol is used for making wine, beer and etc. • As intermediates: - methanol can be oxidised to methanal (HCHO), a chemical feedstock (starting material) for the manufacture of thermosetting plastics such as bakelite. - methanol is used to make methyl methacrylate which is used in the manufacture of another plastic called perspex. • In cosmetics: - ethanol is used as solvent for fragrances in perfumes and aftershave lotions. - polyhydroxyl alcohols (for example, glycerol) are used in moisturising creams. USES OF PHENOLS • Making plastics such as bakelite (phenol-methanal plastic). • The synthesis of cyclohexanol and hexanedioic acid to make nylon 6,6. • Making dyes. • Making antiseptics such as 4-chloro-3,5dimethylphenol which is active ingredient in ‘Dettol’.