* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Incorrect…try again
Survey
Document related concepts
Transcript
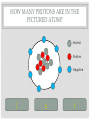
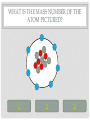
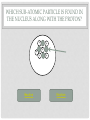
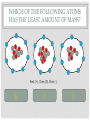
CHEMISTRY QUIZ REVIEW GENERAL SCIENCE – UNIT 3 CLICK TO BEGIN WHICH SUB-ATOMIC PARTICLE IS PICTURED? + + + + + + Neutron Electron Proton INCORRECT...TRY AGAIN. • The neutron is found in the nucleus, however it is neutral. The sub-atomic particle pictured is positive. INCORRECT…TRY AGAIN. • The electron is found orbiting outside of the nucleus. It has a negative charge. CORRECT! • The sub-atomic particle pictured is the proton. It is found in the nucleus and has a positive charge. HOW MANY ELECTRONS ARE IN OXYGEN – 17? 4 8 12 INCORRECT…TRY AGAIN. • Remember, in a neutral atom, the electrons are equal to the protons (atomic number). If there are 4 electrons in the neutral atom, then it only has 4 protons. The element with 4 protons and 4 electrons is Beryllium. INCORRECT…TRY AGAIN. • Remember, in a neutral atom, the electrons are equal to the protons (atomic number). If there are 12 electrons in the neutral atom, then it only has 12 protons. The element with 12 protons and 12 electrons is Magnesium. CORRECT! • In a neutral atom, the electron number is equal to the proton number. The proton number identifies the element. Oxygen is element 8 on the periodic table, therefore it has 8 protons and 8 electrons. WHICH SUB-ATOMIC PARTICLE IDENTIFIES THE ATOM? Proton Neutron Electron INCORRECT…TRY AGAIN. • The neutrons are found in the nucleus with the protons, however, they have no charge and they can differ from atom to atom. INCORRECT…TRY AGAIN. • The electrons are found orbiting outside of the nucleus. They determine reactivity; they do not identify the atom. CORRECT! • The proton identifies the atom. The proton number is the same as the atomic number, which the periodic table is arranged by. WHICH SUB-ATOMIC PARTICLE HAS A NEGATIVE CHARGE - - - Neutron Electron Proton INCORRECT…TRY AGAIN. • The protons are found inside the nucleus and have a positive charge. INCORRECT…TRY AGAIN. • The neutrons are found inside the nucleus and have a neutral charge. CORRECT! • The electron, orbiting around the outside of the nucleus, have a negative charge. WHICH SUB-ATOMIC PARTICLE IS RESPONSIBLE FOR THE ATOM’S MASS? Neutron Electron Proton INCORRECT…TRY AGAIN. • The neutrons are found inside the nucleus with the protons. They contribute to the mass, however, since they are neutral (they have no charge), they do not determine the mass. INCORRECT…TRY AGAIN. • The electrons are found orbiting outside of the nucleus. All of the atom’s mass is found inside the nucleus, therefore, the electrons do not impact the mass of the atom. CORRECT! • The proton, with its positive charge, is responsible for the mass of the atom. To determine which element has the greatest mass, you locate the element with the highest atomic number (# of protons). HOW MANY PROTONS ARE IN THE PICTURED ATOM? Neutral Positive Negative 7 6 5 INCORRECT…TRY AGAIN. • The question is asking for the number of protons. You have identified the number of grey circles, or neutrons. Neutrons are found inside the nucleus with the protons. Neutrons have a neutral charge. INCORRECT…TRY AGAIN. • The question is asking for the number of protons. You have identified the number of blue circles, or electrons. Electrons are found orbiting outside of the nucleus and have a negative charge. CORRECT! • The proton is located inside the nucleus. This eliminates the blue circles. Since protons have a positive charge, they are red. If you count the red circles, there are 5 protons. HOW MANY ELECTRONS DOES AN ATOM HAVE IF IT CONTAINS 17 NEUTRONS AND 19 PROTONS? 17 19 36 INCORRECT…TRY AGAIN. • 36 is found by adding the protons and the neutrons. This number is the mass number, not the number of electrons. INCORRECT…TRY AGAIN. • 17 is the number of neutrons. Remember, in a neutral atom, protons and electrons are equal to one another. CORRECT! • In a neutral atom, electrons are equal to protons. IF the atom has 19 protons, then it must have 19 electrons. WHAT IS THE HYPHENATED NOTATION FOR AN ATOM THAT HAS: 12 PROTONS, 8 NEUTRONS, AND 12 ELECTRONS? Oxygen - 20 Magnesium - 12 Magnesium - 20 Oxygen - 12 INCORRECT…TRY AGAIN. • Hyphenated Notation is the Element Name – Mass # • 8 is the number of neutrons. This does not indicate element name. You need to look at the protons to determine the element name. • Mass # is found by adding protons and neutrons • 20 is the correct mass number. (8+12) INCORRECT…TRY AGAIN. • Hyphenated Notation is the Element Name – Mass # • 8 is the number of neutrons. This does not indicate element name. You need to look at the protons to determine the element name. • Mass # is found by adding protons and neutrons • 12 is only the number of neutrons. You must add the protons to this number. INCORRECT…TRY AGAIN. • Hyphenated Notation is the Element Name – Mass # • You have the right element. • The protons (atomic #) indicate the element name: • 12 protons = Magnesium • The Mass # is found by adding the protons and neutrons. You only used the protons (12). You must add the neutrons (8) to this number. CORRECT! • Hyphenated Notation is the Element Name – Mass # • The protons (atomic #) indicate the element name: • 12 protons = Magnesium • The Mass # is found by adding the protons and neutrons • 12 + 8 = 20 THERE ARE 7 PROTONS IN THE NUCLEUS OF THIS NEUTRAL ATOM. HOW DO YOU KNOW? Neutral Negative Electrons = Protons in a neutral atom Neutrons = Protons in a neutral atom INCORRECT…TRY AGAIN. • In a neutral atom, the number of neutrons (neutral charge) can change. They do not have to equal the number of protons. CORRECT! • In a neutral atom, protons are equal to the number of electrons. THE ATOMIC NUMBER IS EQUAL TO THE NUMBER OF WHICH SUB-ATOMIC PARTICLE? Electron Neutron Proton INCORRECT…TRY AGAIN. • The neutron does not affect the atomic number. The neutron is important in finding the mass number. INCORRECT…TRY AGAIN. • The electron does not affect the atomic number. It determines reactivity. CORRECT! • The atomic number is equal to the number of protons. This number also identifies the element on the periodic table. WHAT IS THE MASS NUMBER OF THE ATOM PICTURED? 7 11 12 INCORRECT…TRY AGAIN. • 7 is the number of electrons. Electrons do not affect the mass number. The atom’s mass is found in it’s nucleus. INCORRECT…TRY AGAIN. • 12 is the number of electrons and one of the particles in the nucleus. Electrons do not affect the mass number. The atom’s mass is found in it’s nucleus. CORRECT! • The mass of the atom is found in the nucleus where the protons and neutrons are located. The mass number is equal to the number of protons added to the number of neutrons. WHAT IS THE NUCLEAR SYMBOL FOR AN ATOM THAT HAS: 15 PROTONS, 6 NEUTRONS, AND 15 ELECTRONS 21 15 P 15 6 C 15 21 P INCORRECT…TRY AGAIN. 15 protons, 6 neutrons, and 15 electrons 15 6 Mass # = protons + neutrons * You used just protons or just electrons C Element Symbol: found by # of protons, find that # on periodic table for element * You used # of neutrons Atomic # = # of protons * You used the # of neutrons INCORRECT…TRY AGAIN. 15 protons, 6 neutrons, and 15 electrons 15 21 Mass # = protons + neutrons * You used just protons or just electrons P Element Symbol: found by # of protons, find that # on periodic table for element * This is correct Atomic # = # of protons •You used the # of neutrons + protons or •You used the # neutrons + electrons CORRECT! 15 protons, 6 neutrons, and 15 electrons Mass # = protons + neutrons 21 15 P Element Symbol: found by # of protons, find that # on periodic table for element Atomic # = # of protons WHAT IS THE ATOMIC NUMBER OF THE PICTURED ATOM? Positive Neutral Negative 4 5 7 INCORRECT…TRY AGAIN. • 4 is the number of neutral particles. These are neutrons. • The atomic number is not equal to the number of neutrons. INCORRECT…TRY AGAIN. • 7 is the number of negative particles. These are electrons. • The atomic number is not equal to the number of electrons. CORRECT! • The atomic number is equal to the number of protons. • Protons have a positive charge (the grey circles) WHAT IS THE HYPHENATED NOTATION FOR THE PICTURED ATOM? Neutral Positive Negative Nitrogen – 9 Boron - 9 Beryllium - 9 INCORRECT…TRY AGAIN. • Hyphenated Notation is the Element Name – Mass # • The protons (atomic #) indicate the element name; these are the RED circles. • You counted the GREY circles, which are neutral and therefore neutrons • The Mass # is found by adding the protons and neutrons • 4+5=9 INCORRECT…TRY AGAIN. • Hyphenated Notation is the Element Name – Mass # • The protons (atomic #) indicate the element name; these are the RED circles. • You counted the BLUE circles, which are negative and therefore electrons • The Mass # is found by adding the protons and neutrons • 4+5=9 CORRECT! • Hyphenated Notation is the Element Name – Mass # • The protons (atomic #) indicate the element name: • Protons are positive (RED circles) • 4 protons = Beryllium • The Mass # is found by adding the protons and neutrons • 4+5=9 WHICH SUB-ATOMIC PARTICLE IS FOUND IN THE NUCLEUS ALONG WITH THE PROTON? + + + + + + Neutron Electron INCORRECT…TRY AGAIN. • Electrons are found outside of the nucleus. CORRECT! • Neutrons are found in the nucleus with the protons. Together the protons and neutrons make up the mass #. WHICH NUCLEAR SYMBOL IS AN ISOTOPE FOR ZINC-51 51 31 Zn 54 30 Zn 51 31 Ga INCORRECT…TRY AGAIN. Isotopes are atoms with the same # of protons (same element), but different #s of neutrons. This means the mass # changes, nothing else! Mass # has to change because it has a different # of neutrons. 51 31 Zn The element name does not change. The atomic number, (# of protons) does not change. If it changes, you have a new element. INCORRECT…TRY AGAIN. Isotopes are atoms with the same # of protons (same element), but different #s of neutrons. This means the mass # changes, nothing else! Mass # has to change because it has a different # of neutrons. 51 31 Ga The element name does not change. By changing the name, you’ve changed the # of protons. The atomic number, (# of protons) does not change. If it changes, you have a new element. CORRECT! Isotopes are atoms with the same # of protons (same element), but different #s of neutrons. This means the mass # changes, nothing else! 54 30 Zn Mass # changes because it has a different # of neutrons. The element name does not change. The atomic number, (# of protons) does not change. HOW MANY PROTONS DOES THE REPRESENTED ATOM HAVE? 25 16 9 S 25 16 INCORRECT…TRY AGAIN. • 25 is the mass #. This is found by adding the # of protons to the # of neutrons Mass # 25 16 S INCORRECT…TRY AGAIN. • 9 is the number of neutrons. This is found by taking the Mass # - the Atomic #. Mass # 25 16 The atomic number S CORRECT! • Protons are equal to the atomic number. They also identify the atom. 25 16 The atomic number S You could find “S” on the periodic table WHICH ELEMENT HAS THE LEAST NUMBER OF PROTONS IN THE NUCLEUS? Calcium #20 on table Tin #50 on table Flourine #9 on table INCORRECT…TRY AGAIN. • The atomic number indicates the number of protons. This element has a higher atomic number, therefore a higher number of protons, then another element listed. INCORRECT…TRY AGAIN. • The atomic number indicates the number of protons. This element has a higher atomic number, therefore a higher number of protons, then another element listed. CORRECT! • The atomic number indicates the number of protons. Since Fluorine has an atomic number of 9, then it has 9 protons. This is the least number of protons. HOW LONG DID IT TAKE TO DEVELOP THE MODERN MODEL OF THE ATOM? A short time A long time INCORRECT…TRY AGAIN. • Remember, as early as Democritus, scientists have been working on the model of the atom for hundreds of years. CORRECT! • It took many scientists a long time to develop the modern model of the atom. (Democritus to present day) WHICH SUB-ATOMIC PARTICLES ARE FOUND IN THE NUCLEUS? electrons and neutrons protons and electrons protons and neutrons INCORRECT…TRY AGAIN. • Electrons are found orbiting outside of the nucleus. • Neutrons are found inside the nucleus. INCORRECT…TRY AGAIN. • Electrons are found orbiting outside of the nucleus. • Protons are found inside the nucleus. CORRECT! • Protons and neutrons are found in the nucleus. Electrons are found orbiting outside of the nucleus. WHAT IS THE NUCLEAR SYMBOL FOR THE PICTURED ATOM? Positive Neutral Negative 9 5 B 9 4 Be 7 9 F INCORRECT…TRY AGAIN. The mass number is the protons and neutrons added together (red and grey) – This is correct The atomic number, identifies the atom (grey circles = protons), you used the red which are neutrons. 9 4 Be Be stands for Beryllium which has 4 protons, but our picture has 5, which is Boron (B). INCORRECT…TRY AGAIN. The mass number is the protons and neutrons added together (red and grey) – You used Blue which are electrons The atomic number, identifies the atom (grey circles = protons), you used the red + grey which equals the mass #. 7 9 F F stands for Fluorine which has 9 protons, but our picture has 5, which is Boron (B). CORRECT! The mass number is the protons and neutrons added together (red and grey) 9 5 The atomic number, identifies the atom (grey circles) B Since the atomic # is 5, you find it on the periodic table WHICH OF THE FOLLOWING ATOMS HAS THE LEAST AMOUNT OF MASS? Red (+), Grey (0), Blue (-) 1 2 3 INCORRECT…TRY AGAIN. • The mass is found in the nucleus. This element has a higher atomic number, therefore a higher number of protons, then another element listed. INCORRECT…TRY AGAIN. • The mass is found in the nucleus. This element has a higher atomic number, therefore a higher number of protons, then another element listed. CORRECT! • There are the least amount of protons in the nucleus, where all of the mass is found. WHAT IS THE ATOMIC NUMBER FOR THE REPRESENTED ATOM? 35 20 35 Ca 55 20 INCORRECT…TRY AGAIN. The mass number is the protons and neutrons added together 35 20 Ca INCORRECT…TRY AGAIN. 55 would be the mass # added to the atomic #. 35 20 Ca CORRECT! 35 20 The atomic number, identifies the atom Ca You could look at the periodic table and find Ca is 20. Atomic number (# of protons) identifies atom. HOW MANY NEUTRONS DOES THE REPRESENTED ATOM HAVE? 51 10 41 Ne 10 51 INCORRECT…TRY AGAIN. 10 would be the atomic #. 51 10 Ne INCORRECT…TRY AGAIN. 51 would be the mass #. 51 10 Ne CORRECT! Mass # - Atomic # = Neutron # The mass number 51 10 The atomic number Ne USING THE REPRESENTED ATOM, 86 IS THE ___________ NUMBER 86 44 The number of electrons Ru The number of protons None of the Above All of the Above The atomic number INCORRECT…TRY AGAIN. The atomic # = proton # = electron #, which are all 44 86 44 Ru CORRECT! It is none of the above. 86 is the mass # The mass number 86 44 Ru HOW MANY ELECTRONS DOES THE REPRESENTED ATOM HAVE? 93 56 93 Ba 56 37 INCORRECT…TRY AGAIN. 93 is the mass #, which is protons + neutrons 93 56 Ba INCORRECT…TRY AGAIN. 37 is the neutron#, which mass # - atomic # 93 56 Ba CORRECT! In a neutral atom, Atomic # = Proton # = Electron # 93 The atomic # 56 Ba IF YOU ADD A NEUTRON TO AN ATOM OF POTASSIUM-27, WHAT RESULTS? A Potassium – 28 atom A Calcium – 27 atom A Potassium – 27 atom INCORRECT…TRY AGAIN. If you add a neutron, you create an isotope. An isotope is an element of the same name, but a different number or neutrons. If you change the neutrons, you must change the mass #, not the element name. To change the element name, you have to change the number of protons. INCORRECT…TRY AGAIN. If you add a neutron, you create an isotope. An isotope is an element of the same name, but a different number or neutrons. If you change the neutrons, you must change the mass #. This answer changed nothing. CORRECT! If you add a neutron, you create an isotope. An isotope is an element of the same name, but a different number or neutrons. If you change the neutrons, you must change the mass # WHICH #’S ARE CORRECT FOR CARBON – 13? Atomic # = 13 Mass # = 6 Protons = 13 Electrons = 7 Neutrons = 13 Atomic # = 6 Mass # = 13 Protons = 6 Electrons = 6 Neutrons = 7 Atomic # = 7 Mass # = 6 Protons = 7 Electrons = 6 Neutrons = 13 INCORRECT…TRY AGAIN. Remember: Atomic # = number of protons, the element on the periodic table Mass # = protons + neutrons Protons = identifies the atom on the periodic table Electrons = the # of protons in a neutral atom Neutrons = mass # - atomic # CORRECT! carbon – 13 Atomic # = 6 Mass # = 13 Protons = 6 Electrons = 6 Neutrons = 7 WHAT DID BOHR CONCLUDE ABOUT THE ATOM? The atom contains negatively charged particles in a sea of positive matter Electrons orbit the nucleus in energy levels Atoms are indivisible INCORRECT…TRY AGAIN. The atom contains negatively charged particles in a sea of positive matter * This was stated by Thomson INCORRECT…TRY AGAIN. Atoms are indivisible * This was stated by Dalton CORRECT! Electrons orbit the nucleus in energy levels WHICH OF THE FOLLOWING IS NOT AN ELEMENT? Neon Oxygen Water INCORRECT…TRY AGAIN. To be an element, it must be on the periodic table. Neon is on the periodic table so it IS an element. INCORRECT…TRY AGAIN. To be an element, it must be on the periodic table. Oxygen is on the periodic table so it IS an element. CORRECT! Water is a compound. It is not found on the periodic table. To be an element, it must be listed on the periodic table. WHAT DID J.J. THOMSON SAY ABOUT THE ATOM? The atom contains negatively charged particles in a sea of positive matter The atom is a void of empty space The atom has a dense, small, positive mass in the center INCORRECT…TRY AGAIN. The atom is a void of empty space * This was stated by Democritus INCORRECT…TRY AGAIN. The atom has a dense, small, positive mass in the center * This was stated by Rutherford CORRECT! The atom contains negatively charged particles in a sea of positive matter DALTON’S MODEL WAS KNOWN AS THE ___________________. Cookie Dough Model Planetary Model Billiard Ball Model INCORRECT…TRY AGAIN. Cookie Dough Model: The atom contains negatively charged particles in a sea of positive matter * This was stated by Thomson INCORRECT…TRY AGAIN. Planetary Model: Electrons travel in orbitals (energy levels) around the nucleus * This was stated by Bohr CORRECT! Billiard Ball – the atom was indivisible YOU HAVE FINISHED THE REVIEW •Good Luck on your Quiz!