* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ppt
Survey
Document related concepts
Transcript
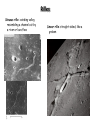
Chapter 10:Planetary surfaces Volcanism and Tectonics Dating craters • Apollo missions returned rock samples from more than half a dozen locations on the Moon’s surface, both maria and highland. • Radioactive dating of these samples provides ages which can then be compared with the number density of craters in each region. Mission Location Sample Age(y) A17 Mare Serenetatis basalt A17 Nectaris highlands 4.3x109 A15 Apennine, PreImbr highland 4.3x109 A15 Imbrium Basin, rim A14 Fra Mauro, Imbrium basin A12 Copernicus: ray+rim A12 A11 #D>10 #D>1 3.3-3.7x109 ≥1000 98000 3.9x109 95 40000 3.9x109 130 ≤0.9x109 10 2000 Oceanus Procellarum basin 3.3x109 20 2000 Mare Tranquilitatis 3.7x109 50 12000 • The numbers in columns 4 and 5 are surface density for craters with diameters >10km (col.4) and >1km (col.5); the surface densities are in units of 10-6/km2 so 2 means 2x10-6. Volcanoes • Volcanism is the process by which material is brought from the interior of the planet to the surface Volcanic structures Cinder cone Lava plain Composite volcano: Mount Hood, Oregon Shield volcanoes: Mauna Loa, Hawaii, and Olympus Mons, Mars Volcanic craters Diatreme Caldera Volcanoes in the Solar System Mare • Lava-covered plains • Dark colour is due to basalt (igneous rock) • Moon mare are exceptionally flat because magma was especially hot (1400-1600 K) and therefore fluid. Mare Crisium on the Moon Caloris Basin on Mars A simple volcano model • Consider a magma chamber, at a depth z embedded in rock of density rR. • Assume the hydrostatic pressure on this chamber is equal to the pressure exerted by the weight of the magma above it: The magma has a lower density rM, and extends a height h above the ground. • The pressure P at the depth of the magma chamber is • So h rR rM z rM P rM g ( z h) r R gz Mauna Loa Calculate the depth of the magma chamber at Mauna Loa (17 km high). The magma has a density of 2770 kg/m3 and the surrounding rock an average density of 3270 kg/m3. h rR rM z rM Outgassing • Volcanoes release gas, as well as molten rock • Can contribute significantly to the composition of the atmosphere. Faults Thrust fault: compression Horsts and Grabens: stretching Wrinkle ridge • Usually found in mare lava plains • Arise from tectonic stresses associated with the cooling and contracting of the lava that flooded the maria Rilles Sinuous rille: winding valley, resembling a channel cut by a river or lava flow Linear rille: straight-sided, like a graben Tectonics Plate tectonics • Rodinia – the giant continent assembled from fragments ~1.2 Gyr ago • began to break up ~750Myr ago • eventually reassembled >200Myr ago “Pangaea” • its breakup led to our continents today • model: bands of alternating colour also alternating magnetic polarity • also crust age increases with distance from rift • observations of Earth’s crust along midocean ridge near Iceland support plate tectonic model Mid-atlantic ridge Tectonic activity on Mars The Acheron Fossae region on Mars, an area of intensive tectonic (continental ‘plate’) activity in the past. Shows how the rifting crosses the older impact crater with at least three alternating horsts and grabens. Break Atmospheric effects Saltation: wind can carry small particles, which bounce on surface and dislodge larger particles Wind Erosion Some regions of Mars’ surface look strikingly like Earth deserts, due to wind erosion. Earth desert Chryse Panitia, Mars Wind streaks As wind sweeps across the Martian plains, dust may be deposited on the leeward sides of craters Dune Fields Sand dunes on Mars Sand dunes in Namiba Geochemical cycles • On planets with atmospheres, surface rock may be tranformed Urey Reaction • A geochemical link between rocks and the atmosphere • On Earth, CO2 from volcanic gases dissolved in rainwater and oceans CO2 H 2O H 2CO3 • This formed a weak carbonic acid, which can to form carbonate rocks. MgSiO3 H 2CO3 MgCO3 SiO2 H 2O • Similarly, living organisms make calcium carbonate shells • Subducted and reconverted to CO2. • This could not occur on Venus (no water), so atmosphere is rich in CO2. Chemistry • The hot atmosphere of Venus (750 K) drives unusual chemical reactions Atmosphere reacts with rocks to produce volatile HCl, HF, sulfuric acid Even mercury and lead may be produced • Any water would have been used up in oxidizing iron minerals or hydrocarbons 2 FeO H 2O Fe2O3 H 2 CH 2 2H 2O CO2 3H 2 Red Mars The red soil of Mars is due to the oxidation of iron atoms in minerals Occurs in the intermittent presence of water The same process that rusts (wet) iron on Earth