* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FDA approves Lymphoseek to help locate lymph nodes in patients
Orphan drug wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Compounding wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
Prescription costs wikipedia , lookup
List of off-label promotion pharmaceutical settlements wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
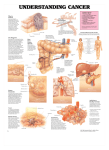
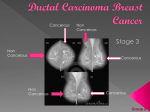
FDA News Release FDA approves Lymphoseek to help locate lymph nodes in patients with certain cancers For Immediate Release March 13, 2013 The U.S. Food and Drug Administration today approved Lymphoseek (technetium Tc 99m tilmanocept) Injection, a radioactive diagnostic imaging agent that helps doctors locate lymph nodes in patients with breast cancer or melanoma who are undergoing surgery to remove tumor-draining lymph nodes. Lymph nodes filter lymphatic fluid that flows from the body’s tissues. This fluid may contain cancer cells, especially if the fluid drains a part of the body containing a tumor. By surgically removing and examining the lymph nodes that drain a tumor, doctors can sometimes determine if a cancer has spread. Lymphoseek is an imaging drug that helps locate lymph nodes; it is not a cancer imaging drug. Lymphoseek is the first new drug used for lymph node mapping to be approved in more than 30 years. Other FDA-approved drugs used for lymph node mapping include sulfur colloid (1974) and isosulfan blue (1981). “Removal and pathological examination of lymph nodes draining a primary tumor is an important diagnostic evaluation for some patients with breast cancer or melanoma,” said Shaw Chen, M.D., deputy director of the Office of Drug Evaluation IV in the FDA’s Center for Drug Evaluation and Research. “To use Lymphoseek, doctors inject the drug into the tumor area and later, using a handheld radiation detector, find lymph nodes that have taken up Lymphoseek’s radioactivity.” Lymphoseek’s safety and effectiveness were established in two clinical trials of 332 patients with melanoma or breast cancer. All patients were injected with Lymphoseek and blue dye, another drug used to help locate lymph nodes. Surgeons subsequently removed suspected lymph nodes for pathologic examination. Confirmed lymph nodes were examined for their content of blue dye and/or Lymphoseek. Results showed Lymphoseek and blue dye had localized most lymph nodes, although a notable number of nodes were localized only by Lymphoseek. The most common side effects identified in clinical trials was pain or irritation at the injection site. Lymphoseek is marketed by Navidea Biopharmaceuticals, Inc. based in Dublin, Ohio. For more information: FDA Approved Drugs: Questions and Answers FDA: Drug Innovation Media Inquiries: Stephanie Yao, 301-796-0394, [email protected] Consumer Inquiries: 888-INFO-FDA The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm343525.htm ###