* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Coombes_ADR_ PM4144 handouts
Survey
Document related concepts
Polysubstance dependence wikipedia , lookup
Compounding wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Orphan drug wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Transcript
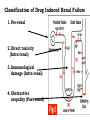
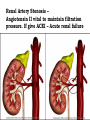
Adverse Drug Events Judith Coombes Neil Cottrell School of Pharmacy, University of Queensland Objectives • What an adverse drug reaction is • Organisations and history • Morbidity and mortality • Classification • Understanding of establishing Causality • Reporting schemes • The role of the Pharmacist Adverse Drug Reaction - ADR • A response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease or for modification of physiological function. WHO 1975 Adverse Drug Event - ADE • • Injury resulting from administration of a drug. • Event is often not solely due to the drug but to events surrounding its use – Unintended administration • Mivacurium (muscle relaxant) instead of metronidazole (antibiotic) – same packaging • Lasix (diuretic) given instead of losec (anti ulcer) – similar name • An entire days worth of iv heparin given in 1 hour Year Event 2000 BC A physician who caused the death of a patient should lose his hand – Babylonian code 950 BC Many drugs were excellent when mingled and many were fatal’ – Homer 1700s ‘They poured drugs of which they knew little into bodies of which they knew less’– Voltaire 1877 British medical association met to investigate the sudden deaths due to chloroform 1937 Sulfonamide elixir containing diethylene glycol given to 353 people – 105 deaths 1952 First book on adverse drug reactions – Myers 1961 Phocomelia due to thalidomide reported McBride Thalidomide • Released 1956 • Used for morning sickness during pregnancy and as sleep aid • Reports of phocomelia in 1961 • Germany 477 reports • Drug withdrawn 1961 • Date Organisation 1962 Food and Drug Administration (FDA) to review all investigational drugs before release – safe as well as effective – USA 1963 Australian Drug Evaluation Committee (ADEC) 1964 Reports of ADRS requested in Australia 1964 Committee on Safety of Drugs - UK 1967 International system to monitor ADRs –WHO 1970 Adverse Drug Reactions Advisory Committee (ADRAC) - Australia Type of ADRs • • Type A – Predictable •Type B – Idiosyncratic/ bizarre Type A • Occur at therapeutic dose – BUT if increase dose ++ will definitely occur • Accentuation of normal drug effect - Bradycardia (↓ heart rate) with a beta blocker – Hypotension (↓ blood pressure) with perindopril • Common – – ~ 75% of all ADRs – ~ 80% of all hospital admissions due to an ADR • High Morbidity • Low Mortality Mechanisms of Type A • Excessive therapeutic effect – Decreased Clearance = drug accumulation – Increased sensitivity target organ • Pharmacological effect at an undesired location – Beta blocker eye drops ↓ heart rate • Another pharmacological action of the drug becomes significant – Anticholinergic effects of tri-cyclic antidepressants Type B (bizzare) • • Not dose related • Unrelated to normal drug effect – Quinine and thrombocytopenia (↓ platelets) – Penicillins and hypersensitivity • Sudden and dramatic in onset • Rare ~ 25% of all ADRs • Low Morbidity • High Mortality Mechanisms of Type B • Immunological – Allergic reactions – anaphylaxis, rash, organ damage (bone marrow, kidneys, liver) • Pharmacogenetic – Factor predisposes the individual to the effect • glucose-6-phosphate dehydrogenase deficiency • ototoxicity with gentamicin Nature of the reactions • Troublesome – Cough, vomiting • Incapacitating – Rash • Life threatening – Organ damage • Bone marrow • Exfoliating rash (skin falls off) • Kidney failure- dialysis Causality • Previous experience • Alternative causes • Patient background • Timing of events • Characteristics of ADR • Stopping the drug • Rechallenge At risk patients • Extremes of age • Patient history – multiple allergy • Genetic polymorphism • Impaired organ function – renal/ hepatic • Polypharmacy – taking lots of drugs • Lack of knowledge – Carbimazole and sore throat Pre-Marketing Surveillance • Human Clinical Trials • Phase I – Volunteers – serious ADRS rare • Phase II – Patients – minor events • Phase III – Targeted for ‘minor events’ • All have small numbers – total ~ 1400 • Need 65,000 participants for ADR 1 in 10,000 Post-Marketing Surveillance • After drug available • Good source for rare reactions • May identify incidence • Spontaneous reporting • Identify risk factors Post-Marketing Study in USA JAMA 2002;287:2215-20 • 548 drugs on market from 1975-1999 • 16 (3%) withdrawn in first 2 years • 45 (8.2%) serious adverse reaction identified. Benefits Spontaneous reporting • All medicines • Whole population • Early warning • Does not affect prescribing habits • Characterise reactions Disadvantages Spontaneous Reporting • Low numbers - 60% reporting rate • No control group for absolute incidence • Causality can be difficult to prove • Problems if long lag for effect • Are they really drug related? What to report • All suspected reactions to NEW DRUGS or drugs of current interest • All suspected drug interactions • Reactions that cause the following – Death – Danger to life – Admission to hospital – Prolongation of hospitalisation – Absence from productive activity – Increased investigational or treatment costs – Birth defects Role of the Pharmacist • Identify drugs with high risk • Patients with risk factors • Ensure appropriate monitoring • Patient education • Identify previous risk/ exposure • Encourage reporting from all • Advise on management of ADRs Respiratory • Amiodarone – ADRAC 2004 17/31 ADR reports were pulmonary – 8 Pulmonary fibrosis – Although onset maybe fast usually it is slow- limit dose • COX 2 inhibitors Rofecoxib and celecoxib • ADR – Increased heart attack and stroke with rofecoxib • Rofecoxib withdrawn • celecoxib caution in heart disease or with risk factors for heart disease. Glitazones (used in type 2 diabetes) Rosiglitazone, pioglitazone • ADR – Increased incidence of heart failure – Increased incidence of heart attacks • Heparin – HITTS – Heparin induced thrombotic thrombocytopenia • Clozapine – Used with monitoring of White Blood Cell count – neutropenia Nephrotoxic agents Which drugs cause renal failure? Which part of the kidney is affected? Classification of Drug Induced Renal Failure 1. Pre-renal 2. Direct toxicity (Intra-renal) 3. Immunological damage (Intra-renal) 4. Obstructive uropathy (Post-renal) 1. Pre-renal Failure Number of different mechanisms : a) Volume depletion e.g. diuretics or cytotoxic therapy b) Prostaglandin inhibition e.g. NSAID’s or c) ACEI (bilateral renal artery disease) Renal Artery Stenosis – Angiotensin II vital to maintain filtration pressure. If give ACEI – Acute renal failure NSAID induced renal impairment Which patients are at risk? Age > 60 yrs Sex F > M Underlying renal disease Concurrent medication esp. if nephrotoxic Decrease in effective circulating blood volume - CCF Cirrhosis nephrotic syndrome volume depletion e.g. diuretics, cytotoxics 2. Direct Toxicity (Intra-renal) a) • ATN : actue tubular necrosis – common (80% of all DIRF). Direct chemical insult to proximal tubule e.g. Aminoglycosides, amphotericin, acyclovir. cyclosporin and cisplatin b) Interstitial damage – papillar necrosis rare (analgesic nephropathy) • Chronic exposure to analgesics or analgesic / antipyretic mixtures (aspirin + paracetamol) • Ingestion > 1 – 3 kg (6 tabs/day for 3 years) 3. Immunological damage (Intra-renal) a) • Immunological damage Allergic vasculitis Rare, part of generalised allergic reaction caused by thiazides, penicillamine • Acute interstitial nephritis Hypersensitivity reaction + extra renal involvement e.g. rash, arthralgia, fever Recovery usually associated with drug removal Causes = gold, penicillins, allopurinol, loop & thiazides • Glomerular damage Immune-mediated – Ag / Ab complex Causes = thiazides, gold, pnicillamine, captopril, NSAID 4. Obstructive uropathy • • • • Retroperitoneal haemorrhage e.g. anticoag’s or fibrinolytic agents Retroperitoneal fibrosis overgrowth fibrous tissue – methyldopa Ureteric obstruction 2 analgesic nephropathy Tubular blockage crystalluria – uric acid / sulphonamides calcium ppt – high serum calcium Hb – drug-induced haemolysis Liver ADRs • Similar findings to viral hepatitis – hepatocellular toxicity • paracetamol overdose, halothane, ??? – drugs can also cause cholestasis • ALP & GGT increase • flucloxacillin, chlorpromazine, ??? – drugs can also induce enzyme production without causing liver damage • phenytoin, barbiturates, acute alcohol intake










































![Is It Making a Difference? [PDF, 8.72MB]](http://s1.studyres.com/store/data/008253928_1-59943b7d1c0ee9fe2fc49012bcc3e283-150x150.png)