* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Adverse Drug Reactions in Children
Survey
Document related concepts
Transcript
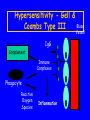
Adverse Drug Reactions in Dentistry (ADRs): Burden of Disease and Special Considerations Michael J. Rieder Section of Paediatric Clinical Pharmacology Children’s Hospital of Western Ontario Division of Clinical Pharmacology Faculty of Medicine & Dentistry University of Western Ontario London, Ontario [email protected] Maria • 6 year old child who had a dental abscess treated in the clinic • Penicillin started 1 week ago • Over the past two days, she has developed fever, malaise and a rash QuickTime™ and a Photo - JPEG decompressor are needed to see this picture. Objectives • Appreciate rate of ADRs • Understand patterns of ADRs to drugs common to dental practice • Appreciate an approach to an ADR associated with dental therapy Perspective on Therapy • God and His Majesty forbid, the fire of the enemy is not half so dangerous as a single drug – M. Platov, 1812 Selective Therapy • Era of selective therapy began in two labs in Europe – Cambridge in 1928 - Sir Alexander Fleming discovery of penicillin – Germany in 1935 - Gerhard Domagk - discovery of sulfanilamide • Demonstration of antimicrobial activity • Serenpedity at work - neither investigator was trying to find an antibiotic Changes in the Paradigm • Demonstration of antimicrobial activity of major importance • Illustration - therapy of Strep. meningitis consisted of rabbit serum, supportive therapy and prayer • Infectious deaths common • Medical paradigm - care, not cure Changes in Care - Consequences • Sulfanilamide activity described in 1935 • Widespread clinical use by 1937 • Major change in clinical care paradigms – In first 10 years of use, 10,000 lives saved in UK among children who would have died of Strep. Infections – Care becomes Cure (Lewis Thomas, Reflections of a Biology Watcher) Elixir of Sulfanilamide Tragedy • Sulfanilamide dissolved in ethylene glycol to improve palatability • Ethylene glycol - a potent nephrotoxin • No pre-marketiug toxicity studies done • Approximately 170 deaths from renal failure, mostly among children • Responsible chemist committed suicide • Major issue in Congress - led to changes that led to current drug regulatory system Introduction • Adverse Drug Reactions are a common and important clinical problem • Seen in 5% of patients treated • Responsible for 5% of all hospital admissions – JAMA 1998; 279: 1200-5 98,000 people in the USA die each year as a result of medical errors ADRs in Dentistry • Relatively little data with respect to ADRs in Dental practice compared to Medical practice • What data is present suggests that overall rates may be similar • No a priori reason to assume different rates ADR Rates • Overall, rate of ADRs in dental patients appears to be similar to adults • Risk appears to relate to known risk factors – Int J Clin Pharmacol Ther 1988; 36: 530-3 Risk Factors for ADRs • History of a previous ADR • Polypharmacy • Impairment of the organs of excretion (hepatic or renal dysfunction) • Extremes of age • Female gender History of ADRs • Elixir of Sulfanilamide Tragedy, 1937 • Chloramphenicol Grey Baby Syndrome, 1950’s • Thallidomide Teratogenicity, 1960’s • Drug substitution errors 1980’s • Ten-fold errors 1990’s • Molecular Misadventures ADR Classification • ADRs often called “drug allergy” • Immune involvement is common, but true drug allergy is relatively rare • Mislabelling leads to therapeutic confusion Hypersensitivity Gell & Coombs Type I Mast Cell IgE Vasodilation Smooth Muscle Contraction Chemotaxis Degranulation Urticaria Bronchoconstriction Hypotension - Shock Inflammation Hypersensitivity Gell & Coombs Type II NK Cell IgG Cell lysis Phagocytosis Phagocyte Complement Removed by Reticuloendothelial System Hypersensitivity - Gell & Blood Coombs Type III Vessel IgG Complement Immune Complexes Phagocyte Reactive Oxygen Species Inflammation Type IV Hypersensitivity Sensitisation Antigen Presenting Cell Immunologic Memory Target Cell Cytotoxic T Cell Cytokines Inflammation Macrophage Cell Destruction Gell and Coombs • Elegant, erudite classification system • Mechanistic • Sadly, does not address the vast majority of ADRs ADR Classifications • A number of schemes have been proposed • Unfortunate and common use of the term allergy • Patterson, DeSwarte and Greenberger (1986) – Predictable – Unpredictable • New England Review of Allergy Proceedings, 1986, 7: 325-42 Predictable ADRs • Predicated on and predictable from the drug’s pharmacology – – – – Side Effects Secondary Effects Interactions Toxicity Unpredictable ADRs • Not known to be related to the drug’s pharmacology – – – – Intolerance Allergic - Pseudoallergic Idiosyncratic Psychogenic Predictable ADRs • Side Effects – Fine tremor associated with inhaled salbutamol (albuterol) • Secondary Effects – Pseudomembranous colitis after lincomycin therapy • Interactions – Bleeding when coumadin and cimetadine are given concurrently • Toxicity – Metabolic acidosis in salicylate overdose Unpredictable ADRs • Intolerance – Intractable vomiting associated with erythromycin therapy • Allergic - Pseudoallergic – Anaphylaxis or urticaria associated with pencillin therapy • Idiosyncratic – Stevens-Johnson Syndrome associated with sulphonamides • Psychogenic – Environmental Hypersensitivity Commonly Used Drugs • • • • • Penicillins Opiates Local Anaesthetics Acetaminophen NSAIDs Penicillins • Can cause all four types of Gell & Coombs reactions • Commonest is Type I (hypersensitivity) • Said to occur in as many as 10% of patients Penicillins • Most common ADRs are skin rash and diarrhoea • Diarrhoea usually self resolving • Rash may be allergic or may be drugdisease interaction Penicillins • Stated incidence of allergy 10% • Actual incidence probably much lower • ADRs described probably represent viral-drug interactions • Can be verified or refuted with skin testing Penicillins • Penicillin skin testing available at selected centres • Testing requires use of both minor and major determinants • Accurate in even small infants • Often deferred until several years after an event Percentage Time Opiates • Commonly used for severe pain • Dose-related respiratory depression in high doses • About 5% of the population develops urticaria on usual doses • NOT an allergy - reflects sensitivity • Crosses the class Local Anaesthetics • Commonly and widely used • Two common problems - inadvertent intravenous injection and allergy • Allergy tends to be unique to class (amide or ester) • Can be tested for Skin Testing • Commonly used • Role is to determine safety, not causation • Hence, usually uses agents of the other class Local Anaesthetic Sensitivity • Ocassionally involves both classes • A considerable problem for the practicing dentist • Benadryl may be used instead modestly effective Acetaminophen • • • • Commonly used Very safe in usual therapeutic doses Only dangerous in overdoses Can occur in setting of repeated suproatherapeutic dosing NSAIDs • Commonly used and increasingly used among children and adolescents • Associated with GI bleeds, gaastrointestinal discomfort • Can be associated with hypersensitivity NSAIDs • Can be cross-class • In this case, may need to use therapeutic alternatives Other Agents • Macrolides - can be associated with vomiting and GI upset • Most common with erythromycin, less common (but not unknown) with newer agents • Clindamycin - diarrhoea more common than with other agents Special Cases • Drug Substitution • 10 fold errors – Unique problem in Paediatrics – More common among certain staff • Drug Errors – Probably more common in children than adults – Again, may be more common among certain staff Medication Errors in a Paediatric ER - One Month’s Experience Type of error Number % Wrong dose Wrong frequency Wrong route Wrong drug Information Other 133 117 7 5 7 2 (49.1%) (43.2%) (2.6%) (1.8%) (2.6%) (0.7%) Total 271 (100%) Medication Errors • Paediatric doses need to be individualized • Knowledge of paediatric doses often much less than optimal • Certain staff - trainees, those unused to working with children, mathematically inept - at higher risk Unique Cases • Special cases arise in which ADR patterns are not the same in children as in adults Cefaclor-associated serum sickness - seen in 1% of children treated, but probably 0.1 to 0.01% of adults -Can J Clin Pharmacol 1999; 6: 197-201 Pre-Marketing Research • Pre-clinical use often includes juvenile animals • Classically, Phase I - III trials include 300 to 5000 patients • Hence, will NOT detect rare but potentially serious events (e.g. most drug-induced hypersensitivities) Limitations of Usual Data • Use of usual data sources for ADR assessment (e.g. CPS) significant • However, usual data sources (e.g. CPS) are poor sources of ADR information – Common events not reported – Rare events over-stated Implications • Novel or serious ADR patterns to new drugs may not be appreciated based on the pre-marketing data available • The CPS may not help you much • Vigilance is important, especially for novel agents Approach to an Undesired Event • Careful Clinical Approach • Evaluation of therapeutic goals – Have we achieved the goal? – If not, how are we going to achieve the goal? – Do we need to revise our goals or do we need to revise our strategy? Clinical Approach to a Possible ADR (I) • History and Physical Examination – Drug, dose, timing, rationale, other events • • • • Analysis of Drug Exposure Differential Diagnosis Obtaining Information Coming to a Clinical Opinion Clinical Approach to a Possible ADR (II) • • • • • Confirmation Communication Treatment Reporting Coping – Patient – Patient-physician relationship References • Patterson R, DeSwartre RD, Greenberger PA et al.: Drug allergy and protocols for management of drug allergies. New England Review of Allergy Proceedings 1896; 7: 325-42 • Rieder MJ: In vitro and in vivo testing for adverse drug reactions. Pediatric Clinics of North America 1997; 44: 93-111 • Gupta A, Waldhauser L: Adverse drug reactions from birth to early childhood. Pediatric Clinics of North America 1997; 44: 79-92 What About Maria? • Stevens-Johnson Syndrome • Pathogenesis related to bioactivation of drug to a reactive intermediate and then (probably) immune propagation • Issues - multi-organ involvement, risk of infection • Therapy - supportive, monitoring for complications, possible use of pulse corticosteroids QuickTime™ and a Photo - JPEG decompressor are needed to see this picture. Take Home Message • • • • Know the drugs that you are using Be vigilant When in doubt, ask When faced with a dilemma, seek expert opinion Acknowledgments • Canadian Institutes of Health Research MRC • Kidney Foundation of Canada • Hospital for Sick Children Foundation • Drs. Gideon Koren, Doreen Matsui, Shinya Ito, Greg Kearns, Bruce Carleton, • Drs. Sanford Cohen, Neil Shear, Ralph Kauffman, Stuart MacLeod