* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Overview of Draft Pharmacovigilance Protocol
Electronic prescribing wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
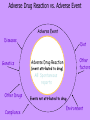
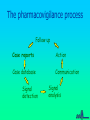
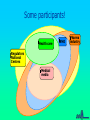
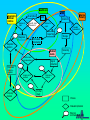
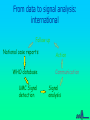
Some Perspectives on a Draft Pharmacovigilance Protocol-reference to HIV/AIDS I Ralph Edwards Identifying ADRs in Africa – Special Challenges: general • • • • • Limited access to health services Limited diagnostic capabilities Over-burdened health care system & staff Significant resource restraints Communication barriers Identifying ADRs in Africa – Special Challenges: HIV/AIDS • Patients need to continue drugs – ADRs common and troublesome • Need to treat ADRs • Combinations of drugs – Which drug? • Disease and complications and ADRs affect multiple overlapping body systems Introduction • Much information and experience in USA/EU, but – Different drug combinations used – Population and disease burden varies • Inadequate infrastructure in place in many 3x5 roll-out countries to monitor safety Definition: Adverse Event vs. Adverse Reaction • Adverse Drug Reaction – A noxious and unintended response to a medicine which occurs at doses normally used in man for treatment, prophylaxis, diagnosis or modification of physiological function. • Adverse Event: – untoward medical occurrence which does not necessarily have to have a causal relationship with the treatment Adverse Drug Reaction vs. Adverse Event Adverse Event Diseases Genetics Diet Other factors Adverse Drug Reaction (event attributed to drug) All Spontaneous reports Other Drugs Compliance Events not attributed to drug Environment Definition: Serious Adverse Event • Any untoward medical occurrence that at any dose results in: – – – – – – Death Is life-threatening Requires or prolongs patient hospitalisation Results in permanent disability/incapacity or is A congenital anomaly/ birth defect Other medically significant event (e.g. blood dyscrasias, seizures) – Does not include NON-serious events that have the POTENTIAL to be SERIOUS if allowed to progress further, nor SEVERE events Objectives of a Basic System • Signal detection for concerns about the safety of drugs • Assessment of signals to evaluate: – causality, – clinical relevance, – frequency and distribution in certain population groups • Communication and recommendations to authorities and public • Appropriate response/action – in terms of drug registration, drug use and/or training and education for professionals and the public • Measurement of outcome of response/action taken – (e.g. reduction in risk of signal, improved drug use, or improved outcome of patients with ADR) The pharmacovigilance process Follow up Case reports Action Case database Communication Signal detection Signal analysis Why Monitor ALL drugs? • Create an awareness of safety issues and drugs • To encourage health professionals to share concerns about drugs • To determine the concerns health professionals have over drugs and their best use – Including interactions • To react to with helpful information to improve therapy • Minimise undue concern about safety of therapies known to cause ADRs – Eg. Anti-retrovirals • Allow for comparison of reporting rates among different therapeutic classes of medicines Elements of the Basic system • Possible general system: – Peripheral health facilities • (spontaneous reporting of drugs used in general medical practice) – Tertiary care facilities and ADR Centre • (Spontaneous reporting and SAEs investigated and intensive monitoring programmes. Special investigations) – Antenatal and delivery clinics • (Pregnancy-related SAE’s and congenital anomalies reported) – Public health Programmes • HIV/AIDS, Malaria etc. Peripheral Health Care Facilities: E.g. health posts, clinics, outreach centres, dispensaries, outpatient departments • Proper prescribing, counseling and administration of meds – Inform patients to return in case of further or ongoing illness – Counsel patients on how to take meds – 1 hour observation post-medication • Completion of SAE form in the event of suspected reaction • Send form to district/state/national level coordinator (depending on infrastructure) • Patients referral to hospital if necessary (with referral note informing of suspected ADR) • Management of non-serious reactions Evaluation/Investigation Team • General or special or geographical (??) – May be comprised of only 1 person • Weekly review of all reports received – Follow-up all/specific SAEs • Home and facility visit if warranted • Return to facility within 2 weeks for investigation • Review ADR forms and Investigation Team report forms • Aggregation into monthly report • Aggregates and individual reports forwarded to national co-ordinator Secondary/Tertiary Care Facilities E.g. Hospitals, health centres (others?) • Investigate any patient attending tertiary care hospitals due to suspected ADR (self-reported, detected in hospital or referred from peripheral health workers) should be investigated • Intensive monitoring in specifically selected facilities – Event monitoring and epidemiological studies Antenatal Clinics and Delivery Services • Report congenital anomalies using SAE reporting form Detection of serious drug reactions intensive (if abnormal lab tests, eg agranulocytosis, interview patient for detailed history) Case-finding or cohort HOSPITAL Laboratory and clinical investigations HEALTH CENTERS PRIVATE CLINICS Generic form Follow-up with detailed report and causality rating, spontaneous DISPENSARIES Shops, traditional healers, other health professionals Roles and Responsibilities • Establish roles and responsibilities of – – – – – – – – – – Patient Clinic staff Traditional Healers and other informal providers District/state/national investigation team National pharmacovigilance co-ordinator Expert safety review panel Malaria control programme Drug regulatory authority Media International agencies (WHO, UMC, etc) Some participants! Health care Regulators National Centres Medical media WHO Pharma industry Health care Regulators/ National Centres Prescription Decision to treat WHO ADR Benefit -harm assessment Collection Decision to report Causality assessment Reporting Diagnosis Regulator’s decision Increased knowledge Medical media Effectiveness risk assessment Pharma industry Storage Screening Signal detection Discussions company regulator Analysis of all evidence Company decision Preliminary analysis Further study Process Evaluation/decision External communication point From data to signal analysis: international Follow up National case reports WHO database UMC Signal detection Action Communication Signal analysis The Importance of Denominators • Denominator: estimated figure of drug use – for estimating frequency of events • Comparisons between drugs difficult and VERY problematic without rates – Often use a comparator/control drug within the system to determine whether lack of signals due to underreporting or real absence of signal Examples of Denominators • Drug procurement figures from central medical stores of MOH • Drug distribution data from EDPs, national drug suppliers/distributors, or manufacturers • Drug records at importation from customs • Notification reports from disease surveillance programmes – ?e.g. HIV/AIDS • Drug procurement records from wholesalers in private sector • Supplementary drug surveys (e.g. treatment seeking behaviour, drug utilisation, or surveys of drug vendors.) Adopting and Adapting – the forms • ADR report form • Evaluation and follow-up form • Special investigations – Public health programmes e.g. HIV/AIDS – Congenital anomaly registers – Etc. • Study protocols Communication: General • All reports must be acknowledged – Reporters must feel a valued part of the system • Useful feedback must be given – Specific to the case if necessary – General, in the form of periodic reports Communication: HIV AIDS • Must allow treating health professionals best information on effectiveness v. risks – Globally and in own population • Give them, and patients, knowledge and confidence to continue therapy in spite of some ADRs • Give information on best avoidance, minimisation, and treatments for ADRs Issues for discussion and consideration (I) • Is this system feasible in your country? – Should it be modified/simplified? • When should you be encouraging reporting of events or reactions • Should you encourage reporting of serious events/reactions only or include non-serious as well? – (This draft does not discourage non-serious reports) • Based on resources, size of country and nature of public health structures– is a special investigation team needed? – Could a ‘generic’ national-level or state-level investigation team suffice? • Can the proposed reporting flow be adapted to your country setting? Issues for discussion and consideration (II) • Should the forms be printed in single or duplicate? If duplicate who will each copy go to? • What should be the timelines for submitting initial reports, investigations reports, aggregate reports? And to whom – supervisor, national coordinator, special investigation team etc. • Which reports to be investigated? All suspected ADRs, clusters ? Unexpected? Unusual? Significantly affecting compliance? Issues for discussion and consideration (III) • Consider the functions and activities of each individual/organisation in your proposed reporting flow • What would be an accurate denominator for drug use for HIV/AIDS treatments and a comparator/s • What do you think are the critical success factors to achieve the system and its objectives? • How can these critical success factors be achieved in your country? Critical Success Factors • • • • • • Literacy of reporters Clearly defined responsibilities Adequate training and education Public awareness of the new medicine Public awareness on reporting safety problems of all medicines Awareness of pharmacovigilance system within informal sector – Community & religious leaders, shopkeepers, traditional healers, community health workers and school teachers • Quality control of laboratories • Open communication between public, health care providers and policy makers – Judicious and pro-active use of the media, professional and general • Presence of national coordinator/s