* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Transition Elements and Their Coordination Compounds
Oxidation state wikipedia , lookup
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Spin crossover wikipedia , lookup
Stability constants of complexes wikipedia , lookup
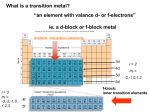
The Transition Elements and Their Coordination Compounds Cu: plumbing Fe: in steel Cr: in automobile parts Au, Ag: jewelry W: light bulb filament Ti: bicycle Pt: auto catalytic converters Zr: nuclear-reactor liners Nitrinol (Ni and Ti used in stents) And many more Transition elements make up the d orbitals (we will cover here) Inner transition metals make up the d and f orbitals The transition elements (d block) and inner transition elements (f block) in the periodic table 1 Example Writing Electron Configurations of Transition Metal Atoms and Ions Write condensed electron configurations for the following: (a) Zr; (b) V3+ (c) Mo3+. (Assume that elements in higher periods behave like those in Period 4.) Note that the general configuration is [noble gas] ns2(n - 1)dx. Recall that in ions the ns electrons are lost first. (a) Zr is the second element in the 4d series: [Kr] 5s24d2. (b) V is the third element in the 3d series: [Ar] 4s23d3. In forming V3+, three electrons are lost (two 4s and one 3d), so V3+ is a d2 ion: [Ar] 3d2 (c) Mo lies below Cr in Group 6B(6), so we expect the same exception as for Cr. Thus, Mo is [Kr] 5s14d5. In forming the ion, Mo loses the one 5s and two of the 4d electrons, so Mo3+ is a d3 ion: [Kr] 4d3 2 Horizontal trends in key atomic properties of the Period 4 elements • Atomic size decreases overall across a period. The d electrons fill inner orbitals, so they shield outer electrons from the increasing nuclear charge very efficiently and the outer 4s electrons are not pulled closer. • Electronegativity usually increases across a period but the transition metals exhibit a relatively small change in electronegativity. Metal in higher oxidation state is more positive has stronger pull on electron is more electronegative • IE1 increase relatively little because the inner 3d electrons shield efficiently and the outer 4s electron experiences only a slightly higher effective nuclear charge. Vertical trends in key properties within the transition elements 3 Trends Within a Group (relative to main-group elements) Atomic Size Increases as expected, from Period 4 to 5. No increase from Period 5 to 6 Lanthanides with buried “4f” sublevel orbitals appear between the 4d (period 5) and 5d (period 6) series An element in Period 6 is separated from the one above it in Period 5 by 32 electrons (ten 4d, six 5p, two 6s, and fourteen 4f). The extra shrinking that results from the increased nuclear charge due to the addition of the fourteen 4f electrons is called the: “Lanthanide Contraction” Order of Sublevel Orbital Filling Electronegativity (EN) – Relative ability of an atom in a covalent bond to attract shared electrons. EN of main-group elements decreases down group Greater size means less attraction by nucleus Greater Reactivity EN in transition elements is opposite the trend in main-group elements EN increases from period 4 to period 5. 4 No change from period 5 to period 6, since the change in volume is small and Zeff increases (f orbital electrons). Transition metals exhibit more covalent bonding and attract electrons more strongly than main-group metals The EN values in the heavy metals exceed those of most metalloids, forming salt-like compounds, such as CsAu and the Au- ion Ionization Energy Main-group elements increase in size down a group, decreasing the 1st ionization energy, making it relatively easier to remove the outer electrons The relatively small increase in size of transition metals, combined with the relatively large increase in nuclear charge (Zeff), results in a general increase in the first ionization energy down a group Density Atomic size (volume) is inversely related to density Across a period densities increase In transition metals the density down a group increases dramatically because atomic volumes change little from Period 5 to Period 6 while nuclear mass increases significantly Period 6 series contains some of the densest elements known: Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold (Density 20 times greater than water, 2 times more dense than lead) Oxidation States 5 • Exhibit more than one • Ionic bonding is more prevalent for the lower oxidation states and covalent bonding is more prevalent for the higher oxidation states. At room temperature TiCl2 is an ionic solid and TiCl4 is a molecular liquid • In high oxidation states atoms have higher charge densities ⇒ polarize electron clouds of non-metals ⇒ covalent bonding • The oxides become less basic as the oxidation state increases TiO is a weak base in water and TiO2 is amphoteric. Color and Magnetism of Compounds Colors of representative compounds of the Period 4 transition metals. sodium chromate titanium oxide scandium oxide nickel(II) nitrate hexahydrate potassium zinc sulfate ferricyanide heptahydrate manganese(II) chloride vanadyl sulfate tetrahydrate dihydrate cobalt(II) chloride hexahydrate copper(II) sulfate pentahydrate • Most main group ionic compounds are colorless because the metal ion has a filled outer level; On the contrary, • Electrons in particular filled d-sublevels can absorb visible wavelengths and move to slightly higher energy d-orbitals. Therefore, many transition metal compounds have striking colors. Exceptions occur when d orbitals are empty or filled. Zn2+: [Ar] 3d10 and Sc3+ or Ti4+ [Ar] 3d0 Formation of Coordination Compounds These are species consisting of a central metal cation (transition metal or main group metal) that is bonded to molecules and or anions called ligands. In order to maintain neutrality in the coordination compound, the complex ion is typically associated with other ions, called counterions. Therefore, 6 Metals ions are Lewis acids, because they accept electrons from Lewis bases. When metal cations combine with Lewis bases, the resulting species is called a complex ion, and the base is called a ligand. 1s0 1s 11s 11s 1 + H H H + + N: H H H F H ammonia sp3 orbitals ammonium ion H N: Hydrogen ion has a vacant 1s orbital. Nitrogen has a full sp 3 orbital. A co-ordinate covalent bond is formed when nitrogen donates its lone e - pair to hydrogen's empty 1s orbital. N H H ammonia H N H H H H H + + B F F N H H F sp2 orbitals B F F B N sp3 orbitals 2pz Boron has a vacant 2p z orbital. Nitrogen has a full sp 3 orbital. A co-ordinate covalent bond is formed when nitrogen donates its lone e - pair to boron's empty 2p z orbital. Ammonia is the Lewis base (electron donor). H+ and BF3 are Lewis acids (electron acceptors). Similarly, transition metals and their cations are Lewis acids. They have vacant orbitals that can accept electron pairs from donor atoms (Lewis bases) forming coordination compounds or complexes. Some examples are shown in the following table. Lewis Acid Cr+3 + Lewis Base 6H2O → Complex [Cr(OH2)6]+3 Dissociation Constants -- Co+3 + 6NH3 → [Co(NH3)6]+3 2.2 × 10-34 Ni+2 Fe Ag+ + + + 4CN5CO 2NH3 → → → [Ni(CN)4]-2 [Fe(CO)5] [Ag(NH3)2]+ 1.0 × 10-31 -6.3 × 10-8 Lewis bases (anions or molecules) bonded to the central Lewis acid are called ligands (Latin = tie or bind). Charged coordination complexes also have counter ions associated with them to satisfy electroneutrality. For example, in [Ag(NH3)2]+NO3-, a nitrate anion is the counter ion. Counter ions are ionically bonded. When dissolved in water, the NO3- ion separates from the complex, but the two NH3 ligands remain firmly bound to the Ag+ ion by covalent bonds. 7 Components of a Coordination Compound When solid complex dissolves in water, the complex ion and the counter ions separate, but ligands remain bound to central atom Coordination Numbers, Geometries, and Ligands Coordination Number (CN) - the number of ligand atoms that are bonded directly to the central metal ion. The coordination number is specific for a given metal ion in a particular oxidation state and compound (6 is the most common, 2 and 4 are often used.) Geometry - the geometry (shape) of a complex ion depends on the coordination number and nature of the metal ion. – See table below Donor atoms per ligand - molecules and/or anions with one or more donor atoms that each donate a lone pair of electrons to the metal ion to form a covalent bond. Importance of Coordination Complexes: Many biologically important substances are 'd'-transition metal coordination compounds that are made up of large organic molecules bound to the metal via coordinate covalent bonds, e.g., hemoglobin (blood protein) is a coordination complex involving Fe. vitamin B-12 is a cobalt complex (cyanocobalamin) phthalocyaine blue is a Cu complex dye used for blue jeans and ink complexing agents are used for water softening (Ca, Mg, Fe removal with EDTA) 8 an antidote for some metal poisoning (BAL) forms complexes with As, Hg, and Cr. Fe-carbonyls are anti-knock gasoline additives Structure of Coordination Compounds: Structures are determined by the coordination number (CN) and VSEPR. Lone pairs of e-'s in d-orbitals have minimal influence on geometry because they are not in the outer shell. Geometries of Various Coordination Numbers CN Geometry 2 Hybridization (nonmetals) Hybridization (transition metals) sp (BeH2) sp [Ag(NH3)2]+ [Cu(CN)2]- sp3 (CH4) sp3 [Zn(CN)4]-2 [Cd(NH3)4]+2 [FeCl4][Co(Br)4]-2 sp3d2 (XeF4) dsp2 [Pd(CO)4]+2 [Pt(NH3)2Cl2] [Ni(CN)4]-2 sp3d (PCl5) d3sp or dsp3 [CuCl5]-3 [Fe(CO)5] [Mn(CO)4NO] [Ni(CN)5]-3 linear 4 109.5º tetrahedral 4 90º 90º Examples (transition metals) square planar a 5 90º or sp3d e e 120º e a 6 triangular bipyramidal e = equitorial position a = axial position 90º 90º octahedral sp3d2 (SF6) d2sp3 or sp3d2 [PtCl6]-2 [Co(NH3)6]+3 [Fe(OH2)6]+2 [Fe(CN)6]-4 [MoF6][CoF6]-3 [Ni(H2O)6]+2 [Mn(CN)6]-4 CN 7 [UF7]-3, [ZrF7]-3, CN 8 [Mo(CN)8]-4, [TaF8]-3 and CN 9 [UCl3 hydrate] are known but rare. 9 Typical CN for some common ions M+ CN M2+ CN M3+ CN + + 2+ 2+ 2+ 2+ 2+ 3+ 3+ 3+ Cu , Au 2,4 Co ,Ni , Cu ,Zn Mn 4,6 Sc , Cr , Co 6 Ag+ 2 Fe2+ 6 Au3+ 4 Types of Ligands and Their Names Rules for writing formulas for the coordination compounds 1. The cation is written before the anion. 2. The charge of the cation(s) is balanced by the charge of the anion(s). 3. For the complex ion, neutral ligands are written before anionic ligands, and the 10 formula for the whole ion is placed in brackets. Relative Strengths of Ligands: Lewis bases differ in their ability to donate electrons to the metal ion. The relative strengths of ligands in coordination complexes has been experimentally determined … I-<Br-< Cl- < F- <OH-<C2O4-2<H2O< SCN-<NH3 <en < NO2-<CN-<CO WEAK STRONG We will come back on this series. Rules for naming complexes 1. 2. 3. 4. 5. 6. The cation is named before the anion. Within the complex ion, the ligands are named, in alphabetical order, before the metal ion. Neutral ligands generally have the molecule name, but there are a few exceptions. Anionic ligands drop the -ide and add -o after the root name. A numerical prefix indicates the number of ligands of a particular type. The oxidation state of the central metal ion is given by a Roman numeral (in parentheses) only if the metal ion can have more than one state. If the complex ion is an anion, we drop the ending of the metal name and add -ate. formula :NH3 H2O name ammonia water :C≡O: :PH3 carbon monoxide phosphine ligand name formula - ammine Cl - aqua F - carbonyl :C≡N: phosphine OH - name ligand name chloride chloro fluoride fluoro cyanide cyano hydroxide hydroxo - :N=O nitric oxide nitrosyl :NO2 nitrite nitro (NO2-) NO3- nitrate nitrato :NO2- nitrite nitrito (ONO-) NH2- amide amido *SO4-2 sulfate sulfato thiocyanate thiocyanato -2 *C2O4 -2 *CO3 *O -2 oxalate oxalato SCN -2 carbonate carbonato *S2O3 thiosulfate thiosulfato oxide oxo C5H5N: pyridine pyridine Example a) What is the systematic name of Na3[AlF6]? The complex ion is [AlF6]3-. Six (hexa-) F- ions (fluoro) as ligands - hexafluoro Aluminum is the central metal atom - aluminate Aluminum has only the +3 ion, so we do not need Roman numerals. ∴ sodium hexafluoroaluminate 11 b) What is the systematic name of [Co(en)2Cl2]NO3? There are two ligands, chlorine and ethylenediamine - dichloro, [bis(ethylenediamine)] The complex is the cation and we have to use Roman numerals for the cobalt oxidation state since it has more than one - (III) The anion, nitrate, is named last. ∴ dichlorobis(ethylenediamine)cobalt(III) nitrate c) What is the formula of tetraamminebromochloroplatinum(IV) chloride? 4 NH3, Br − , Cl − , Pt4+ Cl − ∴ [Pt(NH3)4BrCl]Cl2 d) What is the formula of hexaamminecobalt(III) tetrachloro-ferrate(III)? 6 NH3 Co3+ 4Cl − Fe3+ ∴[Co(NH3)6][FeCl4]3 Chelates chelate with one two ethylenediamine ligand three Notice how the ligand “grabs” the metal from two sides like a claw 12 Coordination Complexes Porphine heme Isomers (same chemical formula but different properties) Coordination isomers occur when the composition of the complex ion changes but not that of the compound [Pt(NH3)4Cl2](NO2)2 and [Pt(NH3)4 (NO2)2]Cl2 Linkage isomers occur when the composition of the complex ions remains the same but the attachment of the ligand donor atom changes [Co(NH3)5(NO2]Cl2 , [Co(NH3)5(ONO]Cl2 13 Geometric isomers (also called cis-trans and sometimes diastereomers) occur when atoms or groups of atoms are arranged differently in space relative to the central metal ion. Beyond cis and trans isomers: facial & meridian isomers and enantiomers CoCl3 ⋅3NH3 Cl H3N Co Cl H3N Cl NH3 facial (fac) Cl H3N Co Cl H3 N NH3 Cl meridian (mer) facial: 3 NH3 and 3 Cl ligands are adjacent (on triangular face) meridian: 3 NH3 ligands in one plane, 3 Cl ligands in a perpendicular plane Optical isomers (also called enantiomers) occur when a molecule and its mirror image cannot be superimposed. Optical isomers have distinct physical properties like other types of isomers, with one exception – the direction in which they rotate the plane of polarized light. 14 Enantiomers: non superimposable mirror images A structure is termed chiral if it is not superimposable on its mirror image ! Two chiral structures: non superimposable mirror images: Enantiomers! Enantiomers NH3 H3N Co Cl H2O Cl H2O NH3 Cl Co NH3 Cl H2O H2O 15 Chirality: absence of a plane of symmetry, possible enantiomers NH3 Cl Co H2O Cl H2O NH3 Achiral (plane of symmetry) NH3 H3N Co Cl H2O Cl H2O NH3 Cl Co NH3 Cl H2O H2O No plane of symmetry: Chiral Which are enantiomers (non-superimposable mirror images) and which are identical (superimposable mirror images)? 16 - Rotating structure I in the cis compound gives structure III, which is not the same as structure II, its mirror image, Image I & Image III are optical isomers - Rotating structure I in the trans compound gives structure III,which is the same as structure II, its mirror image, The trans compound does not have any mirror images 17 Determining the Type of Stereoisomerism PROBLEM: Draw all stereoisomers for each of the following and state the type of isomerism: : PLAN: (b) [Cr(en)3]3+ (en = H2NCH2CH2 NH2) : (a) [Pt(NH3)2Br2] Determine the geometry around each metal ion and the nature of the ligands. Place the ligands in as many different positions as possible. Look for cis-trans and optical isomers. SOLUTION: (a) Pt(II) forms a square planar complex and there are two pair of monodentate ligands - NH3 and Br. These are geometric isomers; they are not optical isomers since they are superimposable on their mirror images. (b) Ethylenediamine is a bidentate ligand. Cr3+ has a coordination number of 6 and an octahedral geometry. Since all of the ligands are identical, there will be no geometric isomerism possible The mirror images are nonsuperimposable and are therefore, optical isomers. 18 Application of VB Theory to Complex Ions Valence Bond Theory of Transition Metal Coordination Compounds: VB theory treats metal to ligand (donor group) bonds as coordinate covalent bonds, formed when a filled orbital of a donor atom overlaps with an empty hybrid orbital on the central metal atom. The molecular geometry is predicted using VSEPR. The theory proposes that the number of metal-ion hybrid orbitals occupied by donor atom lone pairs determine the geometry of the complexes. Lone pairs of electrons are ignored. Since lone pairs are in the inner shell, they are believed to have little effect on molecular geometry. 2-Coordinate Compounds: Linear sp hybridized transition metal complexes: a) Consider [Ag(NH3)2]+ 5p 5s 4d Ag+ = 5s0 4d10 Ag° = 5s1 4d10 sp sp + [Ag(NH3)2] Ag 5p + hybridized :NH3 :NH3 Cu+1 = 4s0 3d10 b) Consider [Cu(CN)2]- Cu° = 4s1 3d10 4p 4s 3d sp sp + [Cu(CN)2] Cu 4p - hybridized + K CN CN :C N: - Four Coordinate Compounds: (Tetrahedral Complexes) a) Consider [ZnCl4]-2 Zn+2 = 4s0 3d10 Zn° = 4s2 3d10 4p 4s 3d sp3 sp3 +2 [ZnCl4] Zn -2 hybridized tetrahedral - - Cl Cl Cl Cl b) Consider [Cd(NH3)4]+2 4d 5s Cd° = 5s2 4d10 5p sp3 sp3 +2 Cd Cd+2 = 5s0 4d10 [Cd(NH3)4] hybridized tetrahedral +2 .. .. .. .. NH3 NH3 NH3 NH3 19 Note that all 4-coordinate complexes of a s0 d10 ion are sp3 hybridized and tetrahedral, e.g., +2 4-coordinate complexes of Group 2B M ions: Zn+2, Cd+2, Hg+2 +1 4-coordinate complexes of Group 1B M ions: Cu+1, Ag+1, Au+1 In four coordinate complexes of s0d8 ions (Ni+2, Pd+2, Pt+2), two molecular geometries are encountered, tetrahedral (sp3) and square planar (dsp2). Consider [NiCl4]-2 3d Ni° = 4s2 3d8 4s 4p sp3 hybridized +2 Ni Ni+2 = 4s0 3d8 tetrahedral [NiCl4] -2 sp3 3d As in previous examples of tetrahedral, sp3 hybridized complexes, the ligand donates electrons to the vacant sp3 hybrid orbitals. This is true when large, weak ligands are present. However, with small, strong ligands, such as CN-, two unpaired electrons in half-filled 3d orbitals are forced to pair up with each other, creating an empty 3d orbital. This gives rise to dsp2 hybridization and the square planar geometry seen in the following examples. Four Coordinate Compounds: (Square Planar Complexes): When very strong ligands are present, four-coordinated d8 metal ions usually form square planar complexes (rather than tetrahedral). Consider [Ni(CN)4]-2 3d Ni° = 4s2 3d8 4s 4p Ni+2 3d 4s 4p dsp2 hybridized [Ni(CN) ]-2 4 square planar Ni+2 = 4s0 3d8 dsp2 3d - 4p - - CN CN- CN CN In dsp2 hybridization, one d-orbital is empty and receives an electron pair from the ligand. Donor atoms are situated on the x and y -axes of the square planar structure. Note that in the term 'dsp2', 'd' precedes 'sp2' indicating that the d-orbital used in hybridization comes from a lower (inner) shell than the 's' and 'p' orbitals. This is called inner shell hybridization. In the previous unit on bonding, we saw terms such as 'sp3d'. In this case, where 'd' follows 'sp3', the d-orbital is from the highest energy level, e.g., recall that PCl5 has sp3d hybridization (triangular bipyramidal geometry). This is called outer shell hybridization. 20 Six Coordinate Complexes (Octahedral): Six ligand donor atoms in an octahedral complex are located at the corners of an octahedron. Valence bond postulates that the orbitals best shaped and best oriented to receive electron pairs from these directions are the dx2-y2 and dz2 orbitals (directed on the x, y, and z-axes). Consider [Fe(CN)6]-3 Fe° = 4s2 3d6 3d Fe 4p 4s +3 3d Fe Fe+3 = 4s0 3d5 4s d2sp3 hybridized 4p +3 octahedral d2sp3 3d [Fe(CN)6] -3 - - - - CN CN- CN CN- CN CN CN- is a strong field donor, forcing inner 3d electrons into a lower spin state and hybridizing inner 3d orbitals, i.e., d2sp3 (inner shell hybridization). H2O, a weaker field donor, is not able to bring about inner shell hybridization and the resulting hybridization is thus sp3d2. Both d2sp3 and sp3d2 complexes are octahedral. Consider [Fe(H2O)6]+3 3d Fe Fe° = 4s2 3d6 sp 3d2 4d 4p 4s Fe+3 = 4s0 3d5 hybridized +3 octahedral sp 3d2 3d [Fe(H2O)6] 4d +3 H2O H2O H2O H2O H2O H2O Metal ions in which two d-orbitals are vacant will form inner shell hybridized d2sp3 octahedral complexes. Metal ions in which two d-orbitals are not vacant will form either outer shell sp3d2 hybridized octahedral complexes (with weak ligands) or inner shell d2sp3 hybridized octahedral complexes with strong ligands (If half-filled d-orbitals are present a low spin state can be forced). Study the following examples and note where inner and outer shell hybridization occur and why. d6 x x Co+3 3d [Co(NH3)6] x x x x 4d 4p 4s x x +3 = an electron pair donated from a ligand x x x x x x strong ligand d2sp 3 3d [CoF6] -3 x x 4d 4p 4s x x x x x x sp 3d2 x x x x weak ligand 21 d5 Mn+2 3d [Mn(CN)6] -4 x x 4d 4p 4s x x x x x x x x x x d2sp 3 d8 Ni+2 3d [Ni(H2O)6] x x 4d 4p 4s +2 x x x x x x x x x x sp 3d2 d3 Cr +3 3d [Cr(NH3)6] +3 x x x x 4d 4p 4s x x x x x x x x d2sp 3 What is color? White light: EM radiation consisting of all λ’s in the visible range. Objects appear colored in white light b/c they absorb certain λ’s and transmit (reflect) other λ’s. The transmitted light enters the eye, hits the retina and the brain perceives a color. • If an object absorbs all λ’s ⇒ black • If an object reflects all λ’s ⇒ white • If an object absorbs all λ’s except for green , the reflected (transmitted) green enters brain and it is interpreted as green. • If an object absorbs only red (complementary of green) the remaining mixture of reflected (transmitted) λ’s is entered the brain and it is interpreted as green. 22 Valence Bond Theory in Coordination Compounds: An Overview Ligands (Lewis base) form coordinate covalent bonds with metal center (Lewis acid) Relationship between hybridization, geometry, and magnetism Inadequate explanation for colors of complex ions e.g., [Cr(H2O)6]3+, [Cr(H2O)4Cl2]+ Crystal Field Theory Basis: purely electrostatic Spherical Field: d orbitals degenerate Overview of d orbitals 23 Splitting of d-orbital energies by an octahedral field of ligands ∆ is the splitting energy Factors affecting magnitude of ∆ 1. Oxidation state of the metal ion 2. Nature of the metal ion 3. Number and geometry of the ligands 4. Nature of the ligands The effect of the ligand on splitting energy 24 The color of [Ti(H2O)6]3+ Effects of the metal oxidation state and of ligand identity on color [V(H2O)6]3+ [V(H2O)6]2+ [Cr(NH3)6]3+ [Cr(NH3)5Cl ]2+ • For a given ligand, the color depends on the oxidation state of the metal ion. • For a given metal ion, the color depends on the ligand. The spectrochemical series 25 Ranking Crystal Field Splitting Energies for Complex Ions of a Given Metal PROBLEM: Rank the ions [Ti(H2O)6]3+, [Ti(NH3)6]3+, and [Ti(CN)6]3- in terms of the relative value of ∆ and of the energy of visible light absorbed. PLAN: The oxidation state of Ti is +3 in all of the complexes so we are looking at the crystal field strength of the ligands. The stronger the ligand, the greater the splitting, and the higher the energy of the light absorbed. SOLUTION: The ligand field strength is CN- > NH3 > H2O, so the relative size of ∆ and energy of light absorbed is [Ti(CN)6]3- > [Ti(NH3)6]3+ > [Ti(H2O)6]3+ High-spin and low-spin complex ions of Mn2+ 26 Identifying Complex Ions as High Spin or Low Spin PROBLEM: PLAN: Iron (II) forms an essential complex in hemoglobin. For each of the 2+ 4two octahedral complex ions [Fe(H2O)6] and [Fe(CN)6] , draw an orbital splitting diagram, predict the number of unpaired electrons, and identify the ion as low or high spin. 2+ The electron configuration of Fe gives us information that the iron has 6d electrons. The two ligands have different field strengths. Draw the orbital box diagrams, splitting the d orbitals into eg and t2g. Add the electrons noting that a weak-field ligand gives the maximum number of unpaired electrons and a high-spin complex and vice-versa. SOLUTION: PE [Fe(CN)6]4- [Fe(H2O)6]2+ 4 unpaired e (high spin) eg t2g -- eg no unpaired e (low spin) -- t2g Orbital occupancy for high-spin and low-spin complexes of d4 through d7 metal ions Splitting of d-orbital energies by a tetrahedral field of ligands Splitting of d-orbital energies by a square planar field of ligands 27 Organometallic compounds: σ and π bonded compounds Ni(CO)4 and Fe(CO)5 Bonding in carbonyls Donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. – M + C O: σ-overlap – M C O: donation of a pair of electrons from a filled d-orbital of metal into vacant antibonding (π*) orbital of CO. M + C M O: C O π-overlap 28 Bonding in alkenes π-electron density of the alkene overlaps with a σ-type vacant orbital on the metal atom C + M C σ - overlap M C C flow of electron density from a filled d-orbital on the metal into the vacant p* antibonding molecular orbital on the carbon atoms C M + C Back-bonding C M C 29






































![[Zn(NH3)4]SO4 [Cr(NH3)5Cl]Cl2 [Co(en)2Br2]2SO4](http://s1.studyres.com/store/data/000163042_1-5a721100d3f3517024b8f44b530a31a4-150x150.png)