* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2.02.26 Percutaneous LeftAtrial Appendage Closure
Coronary artery disease wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
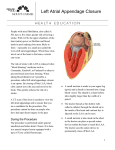
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Percutaneous Left Atrial Appendage Closure Devices for Stroke Prevention in Atrial Fibrillation Number Effective Date Revision Date(s) Replaces 2.02.26 August 1, 2016 01/01/17; 07/12/16; 12/08/15; 09/08/14; 05/13/13;05/08/12 N/A Policy [TOP] The use of a device with U.S. Food and Drug Administration (FDA) approval for percutaneous left atrial appendage closure (e.g., the Watchman) may be considered medically necessary for the prevention of stroke in patients with atrial fibrillation when the following criteria are met: There is an increased risk of stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc score and systemic anticoagulation therapy is recommended; AND The long-term risks of systemic anticoagulation outweigh the risks of the device implantation (see Policy Guidelines). The use of a device with FDA approval for percutaneous left atrial appendage closure (e.g., the Watchman) for stroke prevention in patients who do not meet the above criteria is considered investigational. The use of other percutaneous left atrial appendage closure devices, including but not limited to the Lariat, PLAATO, and Amplatzer devices, for stroke prevention in patients with atrial fibrillation is considered investigational. Related Policies [TOP] None Policy Guidelines [TOP] The balance of risks and benefits associated with implantation of the Watchman device for stroke prevention, as an alternative to systemic anticoagulation with warfarin, must be made on an individual basis. Bleeding is the primary risk associated with systemic anticoagulation. A number of risk scores have been developed to estimate the risk of significant bleeding in patients treated with systemic anticoagulation. An example is the HAS-BLED score, which has validated to assess the annual risk of significant bleeding in patients with AF treated with warfarin (Pisters et al., 2010). The score ranges from 0 to 9, based on a number of clinical characteristics (see Table PG1). Table PG1: Clinical Components of the HAS-BLED Bleeding Risk Score (Pisters et al., 2010) Letter H A S B L E D Clinical Characteristic Hypertension Abnormal renal and liver function (1 point each) Stroke Bleeding Labile INRs Elderly (>65) Drugs or alcohol (1 point each) Points Awarded 1 1 or 2 1 1 1 1 1 or 2 INR: international normalized ratio. Risk of major bleeding in patients with scores of 3, 4, and 5 has been reported at 3.74 per 100 patient-years, 8.70 per 100 patient-years, and 12.5 per 100 patient-years, respectively. Scores of 3 or greater are considered to be associated with high risk of bleeding, potentially signaling the need for closer monitoring of patients for adverse risks, closer monitoring of international normalized ratio, or differential dose selections of oral anticoagulants or aspirin (January et al, 2014). Coding 33340 33999 93799 0281T CPT Percutaneous transcatheter closure of the left atrial appendage with endocardial implant, including fluoroscopy, transseptal puncture, catheter placement(s), left atrial angiography, left atrial appendage angiography, when performed, and radiological supervision and interpretation (new code effective 1/1/17) Unlisted procedure, cardiac surgery Unlisted cardiovascular service or procedure Percutaneous transcatheter closure of the left atrial appendage with implant, including fluoroscopy, transseptal puncture, catheter placement(s), left atrial angiography, left atrial appendage angiography, radiological supervision and interpretation Description [TOP] Stroke prevention in atrial fibrillation (AF) is an important goal of treatment. Treatment with anticoagulant medications is the most common approach to stroke prevention. Most embolic strokes originate from the left atrial appendage; therefore, occlusion of the left atrial appendage may offer a nonpharmacologic alternative to anticoagulant medications for the prevention of stroke in patients with AF. Multiple percutaneously deployed devices are being investigated for left atrial appendage closure. There is one left atrial appendage (LAA) occlusion device with approval from the U.S. Food and Drug Administration (FDA) for stroke prevention in patients with AF, the Watchman™ device. For individuals who have AF who are at increased risk for embolic stroke who receive the Watchman percutaneous LAA closure (LAAC) device, the evidence includes 2 randomized controlled trials (RCTs) and metaanalyses of these trials. Relevant outcomes are overall survival, morbid events, and treatment-related morbidity. The most relevant evidence comes from 2 industry-sponsored RCTs that compared the Watchman device with anticoagulation. One trial reported noninferiority on a composite outcome of stroke, cardiovascular/unexplained death, or systemic embolism after 2 years of follow-up, with continued benefits with the Watchman device after 4 years of follow-up. The second trial did not demonstrate noninferiority for the same composite outcome, but did demonstrate noninferiority of the Watchman device to warfarin for late ischemic stroke and systemic embolization. Patient-level meta-analyses of the 2 trials reported that the Watchman device is noninferior to warfarin on the composite outcome of stroke, systemic embolism, and cardiovascular death. Also, the Watchman was associated with a higher periprocedural risk of bleeding and ischemic stroke but a lower risk of hemorrhagic stroke over the long term. The published evidence indicates that the Watchman device is efficacious in preventing stroke for patients with AF who are at increased risk for embolic stroke. When it is determined on an individualized basis that the long-term risk of systemic anticoagulation exceeds the procedural risk of device implantation, the net health outcome will be improved. The evidence is sufficient to determine qualitatively that the technology results in a meaningful improvement in the net health outcome. For individuals who have AF who are at increased risk for embolic stroke who receive a percutaneous LAAC device other than the Watchman device (eg, the Lariat, Amplatzer, and PLAATO devices), the evidence includes uncontrolled case series. Relevant outcomes are overall survival, morbid events, and treatment-related morbidity. Case series of these devices have reported high procedural success, but also numerous complications. In addition, these devices do not have FDA approval for LAAC. The evidence is insufficient to determine the effects of the technology on health outcomes. Background Stroke is the most serious complication of atrial fibrillation (AF). The estimated incidence of stroke in nontreated patients with AF is 5% per year. Stroke associated with AF is primarily embolic in nature, tends to be more severe than the typical ischemic stroke, and causes higher rates of mortality and disability. As a result, stroke prevention is one of the main goals of AF treatment. Stroke in AF occurs primarily as a result of thromboembolism from the left atrium. The lack of atrial contractions in AF leads to blood stasis in the left atrium, and this low flow state increases the risk for thrombosis. The area of the left atrium with the lowest blood flow in AF, and, therefore, the highest risk of thrombosis, is the left atrial appendage (LAA). It has been estimated that 90% of leftatrial thrombi occur in the LAA. The main treatment for stroke prevention in AF is anticoagulation, which has proven efficacy. The risk for stroke among patients with AF is stratified on the basis of several factors. A commonly used score, the CHADS2 score, assigns 1 point each for the presence of heart failure, hypertension, age 75 years or older, diabetes, or prior stroke or transient ischemic attack. The CHADS2-VASc score includes sex, more age categories, and the presence of vascular disease, in addition to the risk factors used in the CHADS2 score. Warfarin is the predominant agent in clinical use. A number of newer anticoagulant medications, including dabigatran, rivaroxaban, and apixaban, have recently received U.S. Food and Drug Administration (FDA) approval for stroke prevention in nonvalvular AF and have demonstrated noninferiority to warfarin in clinical trials. While anticoagulation is effective for stroke prevention, there is an increased risk of bleeding. Also, warfarin requires frequent monitoring and adjustments, as well as lifestyle changes. Dabigatran does not require monitoring. However, unlike warfarin, the antithrombotic effects of dabigatran are not reversible with any currently available hemostatic drugs. Guidelines from the American College of Chest Physicians recommend the use of oral anticoagulation for patients with AF who are at high risk of stroke (i.e., CHADS2 score ≥ 2), with more individualized choice of antithrombotic therapy in patients with lower stroke risk.(1) Bleeding is the primary risk associated with systemic anticoagulation. A number of risk scores have been developed to estimate the risk of significant bleeding in patients treated with systemic anticoagulation. An example is the HAS-BLED score, which has validated to assess the annual risk of significant bleeding in patients with AF treated with warfarin.(2) The score ranges from 0 to 9, based on a number of clinical characteristics, including the presence of hypertension, renal and liver function, history of stroke, bleeding, labile international normalized ratios (INRs), age, and drug/alcohol use. Scores of 3 or greater are considered to be associated with high risk of bleeding, potentially signaling the need for closer monitoring of the patient for adverse risks, closer monitoring of INRs, or differential dose selections of oral anticoagulants or aspirin.(3) Surgical removal, or exclusion, of the LAA is often performed in patients with AF who are undergoing open heart surgery for other reasons. Percutaneous LAA closure devices have been developed as a nonpharmacologic alternative to anticoagulation for stroke prevention in AF. The devices may prevent stroke by occluding the LAA, thus preventing thrombus formation. Several versions of LAA occlusion devices have been developed. The WATCHMAN™ left atrial appendage system (Boston Scientific, Maple Grove, MN) is a self-expanding nickel titanium device. It has a polyester covering and fixation barbs for attachment to the endocardium. Implantation is performed percutaneously through a catheter delivery system, using venous access and transseptal puncture to enter the left atrium. Following implantation, patients are anticoagulated with warfarin or alternative agents for approximately 1 to 2 months. After this period, patients are maintained on antiplatelet agents (i.e., aspirin and/or clopidogrel) indefinitely. The Lariat® Loop Applicator is a suture delivery device that is intended to close a variety of surgical wounds in addition to left atrial appendage closure. The Cardioblate® closure device developed by Medtronic is currently being tested in clinical studies. The Amplatzer® cardiac plug (St. Jude Medical, Minneapolis, MN), is FDA-approved for closure of atrial septal defects but not LAA closure device. A second-generation device, the Amplatzer Amulet, has been developed. The Percutaneous LAA Transcatheter Occlusion device (eV3, Plymouth, MN) has also been evaluated in research studies but has not received FDA approval. Regulatory Status In 2009, the Watchman Left Atrial Appendage Closure Technology (Boston Scientific, Marlborough, MA) was originally considered by the U.S. Food and Drug Administration (FDA) for approval based on the results the results of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation (PROTECT-AF) randomized controlled trial (RCT). The device underwent three panel reviews before it was approved by FDA through the premarket approval process on March 13, 2015.(4) This device is indicated to reduce the risk of thromboembolism from the left atrial appendage (LAA) in patients with nonvalvular atrial fibrillation who: Are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy; Are deemed by their physicians to be suitable for warfarin; and Have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin. FDA product code: NGV. At least two other devices have been studied for LAA occlusion, but are not approved in the United States for percutaneous closure of the LAA. In 2006, The Lariat® Loop Applicator device (SentreHEART, Redwood City, CA), a suture delivery system, was cleared for marketing by the FDA through the 510(k) process. The intended use is to facilitate suture placement and knot tying in surgical applications where soft tissues are being approximated or ligated with a pretied polyester suture. The Amplatzer Amulet® device (St. Jude Medical, Plymouth, MN) has a CE approval in Europe for LAA closure, but is not currently approved in the United States for any indication. Scope [TOP] Medical policies are systematically developed guidelines that serve as a resource for Company staff when determining coverage for specific medical procedures, drugs or devices. Coverage for medical services is subject to the limits and conditions of the member benefit plan. Members and their providers should consult the member benefit booklet or contact a customer service representative to determine whether there are any benefit limitations applicable to this service or supply. This medical policy does not apply to Medicare Advantage. Benefit Application [TOP] N/A Rationale [TOP] Populations Individuals: With atrial fibrillation who are at increased risk for embolic stroke Individuals: With atrial fibrillation who are at increased Interventions Interventions of interest are: Watchman percutaneous left atrial appendage closure device Interventions of interest are: Percutaneous left atrial appendage closure device Comparators Comparators of interest are: Anticoagulation Comparators of interest are: Anticoagulation Outcomes Relevant outcomes include: Overall survival Morbid events Treatment-related morbidity Relevant outcomes include: Overall survival Morbid events risk for embolic stroke other than the Watchman device Treatment-related morbidity This policy was created in April 2011 and has been updated periodically with literature search of the MEDLINE database. The most recent update with literature review covers the period of through April 30, 2016. Assessment of efficacy for therapeutic interventions such as the left atrial appendage (LAA) closure devices involves a determination of whether the intervention improves health outcomes. The optimal study design for this purpose is a randomized controlled trial (RCT) that includes clinically relevant measures of health outcomes. Intermediate outcome measures, also known as surrogate outcome measures, may also be adequate if there is an established link between the intermediate outcome and true health outcomes. Nonrandomized comparative studies and uncontrolled studies can sometimes provide useful information on health outcomes, but are prone to biases. For the use of LAA closure devices, the appropriate comparison group could be oral anticoagulation, no therapy (for patients who have prohibitive risk for oral anticoagulation), or open surgical repair. The evidence on the efficacy of LAA closure devices consists of numerous case series of various occlusion devices, and 2 published RCTs of the Watchman™ device that compared LAA closure with warfarin anticoagulation. Evidence on each different device will be reviewed separately, because the devices are not similar in design, and each may have its own unique considerations. Watchman Device The Watchman device is intended as an alternative to anticoagulation for patients with atrial fibrillation (AF) who are at increased risk for embolic stroke. RCTs comparing the Watchman device to anticoagulation are essential for determining efficacy of the device. Nonrandomized studies and case series may offer additional evidence on populations included in the RCTs, durability of effect, and/or adverse events. This evidence review will include RCTs and systematic reviews of RCTs for efficacy, and will review select nonrandomized studies and case series that offer additional information of different populations, durability, and/or adverse events. The review of the evidence related to the efficacy of the Watchman device is based, in part, on a Blue Cross Blue Shield Association TEC Assessment developed in June 2014, which evaluated use of the Watchman device for patients who were eligible and ineligible for anticoagulation therapy and determined that it does not meet Technology Evaluation Criteria.(5) The TEC assessment made the following conclusions about the use of LAA closure in patients without contraindications to anticoagulation: We identified two randomized controlled trials (RCTs) and one case series evaluating the Watchman™ device. The RCTs were noninferiority trials and compared LAAC with anticoagulation. The first trial showed a lower rate of a composite outcome (stroke, death, and embolism) in patients receiving LAAC [left atrial appendage closure] and met noninferiority criteria compared with anticoagulation, but FDA [Food and Drug Administration] review noted problems with patient selection, potential confounding with other treatments, and losses to follow-up. The second trial, which incorporated the first trial’s results as a discounted informative prior in a Bayesian analysis, showed similar rates of the same composite outcome but did not meet noninferiority criteria. The second trial met its second principal outcome noninferiority criteria in 1 of 2 analyses and a performance goal for short-term complication rate. When assessing the results of both trials, the relative performance of LAAC and anticoagulation is uncertain. Although the Watchman device and other LAA closure devices would ideally represent an alternative to oral anticoagulation for the prevention of stroke in patients with AF, during the postimplantation period, the device may be associated with increased thrombogenicity and, therefore, anticoagulation is used during the periprocedural period. Most studies evaluating the Watchman device have included patients who are eligible for anticoagulation. Two individual RCTs, the PROTECT AF and PREVAIL trials, have evaluated the Watchman device for stroke prevention in patients with AF who are at increased risk for embolic stroke. These trials are reviewed in depth next, along with meta-analyses of these studies. Meta-Analyses A number of meta-analyses have been performed that combine results of the available RCTs.(6-11) The most rigorous of them is a patient-level meta-analysis by Holmes et al.(8) This study included patient-level data from the industry-sponsored PROTECT AF and PREVAIL trials, described below, together with both studies’ continued access registries.(8) The PROTECT AF and PREVAIL registries were designed to include patients with similar baseline characteristics as their respective RCTs. The meta-analysis included 2406 patients, 1877 treated with the Watchman device and 382 treated with warfarin alone. Mean patient follow-up durations were 0.58 years and 3.7 years, respectively, for the PREVAIL continued access registry and the PROTECT AF continued access registry. In a meta-analysis of 1114 patients treated in the RCTs, compared with warfarin, LAAC met the study’s noninferiority criteria for the primary composite efficacy end point of all-cause stroke, systemic embolization, and cardiovascular death (hazard ratio [HR], 0.79, 95% confidence interval [CI], 0.52 to 1.2; p=0.22). All-cause stroke rates did not differ significantly between groups (1.75 per 100 patient-years for LAAC vs 1.87 per 100 patientyears for warfarin; HR=1.02; 95% CI, 0.62 to 1.7; p=0.94). However, LAAC-treated patients had higher rates of ischemic stroke (1.6 events per 100 patient-years vs 0.9 events per 100 patient-years; HR=1.95, p=0.05) when procedure-related strokes were included, but had lower rates of hemorrhagic stroke (0.15 events per 100 patientyears vs 0.96 events per 100 patient-years; HR=0.22; 95% CI, 0.08 to 0.61; p=0.004). A second patient-level meta-analysis of the 2 RCTs evaluated bleeding outcomes.(11) There were a total of 54 episodes of major bleeding, with the most common types being gastrointestinal (GI) bleed (31/54 [57%]) and hemorrhagic stroke (9/54 [17%]). On combined analysis, the rate of major bleeding episodes over the entire study period did not differ between groups. There were 3.5 events per 100 patient-years in the Watchman group compared with 3.6 events per 100 patient-years in the anticoagulation group, for a rate ratio (RR) of 0.96 (95% CI, 0.66 to 1.40; p=0.84). However, there was a reduction in bleeding risk for the Watchman group past the initial periprocedural period. For bleeding events occurring more than 7 days postprocedure, the event rates were 1.8 per 100 patient-years in the Watchman group compared with 3.6 per 100 patient-years in the anticoagulation group (RR=0.49; 95% CI, 0.32 to 0.75; p=0.01). For bleeding events occurring more than 6 months postprocedure (the time at which antiplatelet therapy is discontinued for patients receiving the Watchman device), the event rates were 1.0 per 100 patient-years in the Watchman group compared with 3.5 per 100 patient-years in the anticoagulation group (RR=0.28; 95% CI, 0.16 to 0.49; p<0.001). Randomized Controlled Trials PROTECT-AF Trial The first RCT published was the PROTECT AF study,(12) which was a randomized, unblinded trial that evaluated the noninferiority of an LAA closure device compared with warfarin for stroke prevention in AF. The trial randomized 707 patients from 59 centers in the United States and Europe to the WATCHMAN® device or warfarin treatment in a 2:1 ratio. Mean follow-up was 18±10 months. The primary efficacy outcome was a composite endpoint of stroke (ischemic or hemorrhagic), cardiovascular or unexplained death, or systemic embolism. There was also a primary safety outcome, a composite endpoint of excessive bleeding (intracranial or gastrointestinal [GI] bleeding) and procedure-related complications (pericardial effusion, device embolization, procedure-related stroke). The primary efficacy outcome occurred at a rate of 3.0 per 100 patient-years in the LAA closure group compared with 4.9 per 100 patient-years in the warfarin group (rate ratio [RR], 0.62; 95% credible interval [CrI], 0.35 to 1.25). Based on these outcomes, the probability of noninferiority was greater than 99.9%. For the individual components of the primary outcome, cardiovascular/unexplained death and hemorrhagic stroke were higher in the warfarin group. In contrast, ischemic stroke was higher in the LAA closure group at 2.2 per 100 patient-years compared with 1.6 per 100 patient-years in the warfarin group (RR=1.34; 95% CrI, 0.60 to 4.29). The primary safety outcome occurred more commonly in the LAA closure group, at a rate of 7.4 per 100 patientyears compared with 4.4 per 100 patient-years in the warfarin group (RR=1.69; 95% CrI, 1.01 to 3.19). The excess in adverse event rates for the LAA closure group was primarily the result of early adverse events associated with placement of the device. The most frequent type of complication related to LAA closure device placement was pericardial effusion requiring intervention, which occurred in 4.8% of patients (22/463). Longer term follow-up from the PROTECT AF study was reported by Reddy et al. in 2013.(13) At a mean followup of 2.3 years, the results were similar to the initial report. The relative risk for the composite primary outcome in the Watchman group compared with anticoagulation was 0.71, and this met non-inferiority criteria with a confidence of greater than 99%. Complications were more common in the Watchman group, with an estimated rate of 5.6%/year in the Watchman group compared with 3.6%/year in the warfarin group. Outcomes through 4 years of follow-up were reported by Reddy et al. in 2014.9 Mean follow-up was 3.9 years in the LAA closure group and 3.7 years in the warfarin group. In the LAA closure group, warfarin was discontinued in 345 of 370 patients (93.2%) by the 12 month follow-up evaluation. During the follow-up period, the relative risk for the composite primary outcome in the Watchman group compared with anticoagulation was 0.60 (8.4% in the device group vs. 13.9% in the anticoagulation group; 95% CrI, 0.41 to 1.05), which met the noninferiority criteria with a confidence of greater than 99.9%. Fewer hemorrhagic strokes occurred in the Watchman group (0.6% vs. 4.0%; RR=0.15; 95% CrI, 0.03 to 0.49), and fewer cardiovascular events occurred in the Watchman group (3.7% vs. 0.95%; RR=0.40; 95% CrI, 0.23 to 0.82). Rates of ischemic stroke did not differ significantly between groups, but Watchman group patients had lower all-cause mortality than anticoagulation group patients (12.3% vs. 18.0%; HR=0.66; 95% CI: 0.45 to 0.98; p=0.04). Alli et al. reported quality-of-life parameters, as measured by change in scores on the Short-Form 12-Item Health Survey from baseline to 12-month follow up, for a subset of 547 subjects in the PROTECT AF study. (15) For the subset of PROTECT AF subjects included in the present analysis, at baseline, control group subjects had a higher mean CHADS2 score (2.4 vs. 2.2; p=0.052) and were more likely to have a history of coronary artery disease (49.5% vs. 39.6%; p=0.028). For subjects in the Watchman group, the total physical score improved in 34.9% and was unchanged in 29.9%; for those in the warfarin group, the total physical score improved in 24.7% and was unchanged in 31.7% (p=0.01). PREVAIL Trial A second RCT, the PREVAIL trial, was conducted after the 2009 FDA decision on the Watchman™ device to address some of the limitations of the PROTECT AF study, including its inclusion of patients with low stroke risk (CHADS2 scores of 1), high rates of adjunctive antiplatelet therapy use in both groups, and generally poor compliance with warfarin therapy in the control group. Results from the PREVAIL trial were initially presented in FDA documentation(16), and published in peer-reviewed form by Holmes et al in 2014.(17) In the PREVAIL trial, 461 subjects enrolled at 41 sites were randomized in a 2:1 fashion to either the Watchman™ device or control, which consisted of either initiation or continuation of warfarin therapy with a target international normalized ratio (INR) of 2.0 to 3.0. Subjects had non-valvular AF and required treatment for prevention of thromboembolism based on a CHADS2 score of 2 or higher (or ≥1 with other indications for warfarin therapy based on American College of Cardiology/American Heart Association/European Society of Cardiology guidelines) and were eligible for warfarin therapy. In the device group, warfarin and low-dose aspirin were continued until 45 days postprocedure; if a follow-up echocardiogram at 45 days showed occlusion of the LAA, warfarin therapy could be discontinued. Subjects who discontinued warfarin were treated with aspirin and clopidogrel for 6 months postdevice implantation and with 325 mg aspirin indefinitely after that. Three noninferiority primary efficacy end points were specified: (1) occurrence of ischemic or hemorrhagic stroke, cardiovascular or unexplained death, and systemic embolism (18 month rates); (2) occurrence of late ischemic stroke and systemic embolization (beyond 7 days post-randomization, 18-month rates); and (3) occurrence of allcause death, ischemic stroke, systemic embolism, or device- or procedure-related events requiring open cardiac surgery or major endovascular intervention (e.g., pseudoaneurysm repair, arteriovenous fistula repair, or other major endovascular repair) occurring within 7 days of the procedure or by hospital discharge, whichever was later. The 18-month event rates were determined using Bayesian statistical methods to integrate data from the PROTECT-AF study. All patients had a minimum follow-up of 6 months. For randomized subjects, mean follow-up was 11.8 months and median follow-up was 12.0 months (range, 0.03-25.9 months). The first primary end point, the 18-month modeled RR between the device and control groups was 1.07 (95% CrI, 0.57 to 1.89). Because the upper bound of the 95% CrI was above the preset noninferiority margin of 1.75, the noninferiority criteria were not met. For the second primary end point of late ischemic stroke and systemic embolization, the 18-month RR between the device and control groups was 1.6 (95% CrI, 0.5 to 4.2), with an upper bound of the 95% CrI above the preset noninferiority margin of 2.0. The rate difference between the device and control groups was 0.005 (95% CrI, -0.019 to 0.027). The upper bound of the 95% CrI was lower than the noninferiority margin of 0.0275, so the noninferiority criterion was met for the rate difference. For the third primary end point, major safety issues, the noninferiority criterion was met. Nonrandomized Studies Numerous case series and nonrandomized studies have been published.(18-22) A few of them are notable in that they were conducted in patients not eligible for anticoagulation, a population not included in PROTECT AF and PREVAIL. Reddy et al conducted a multicenter, prospective, nonrandomized trial to evaluate the safety and efficacy of LAAC with the Watchman device in patients with nonvalvular AF with a CHADS 2 score 1 or higher who were considered ineligible for warfarin.(23) Postimplantation, patients received 6 months of clopidogrel or ticlopidine and lifelong aspirin therapy. Thirteen (8.7%) patients had a procedure- or device-related serious adverse event, most commonly pericardial effusion (3 patients). Over a mean 14.4 months of follow-up, all-cause stroke or systemic embolism occurred in 4 patients. Chun et al compared the Watchman device to the Amplatzer cardiac plug among patients with nonvalvular AF, who were at high risk for stroke and had a contraindication to or were not willing to accept oral anticoagulants.(24) Eighty patients were randomized to left atrial appendage occlusion with the Watchman or the Amplatzer device. After device implantation, either preexisting oral anticoagulation therapy or dual platelet inhibition with aspirin and clopidogrel was continued for 6 weeks. There were no statistically significant differences in procedure time, fluoroscopy time, or major safety events between the 2 groups. At a median 364 days of follow-up, there were no cases of stroke/transient ischemic attack or other bleeding complications. The EWOLUTION Watchman registry is intended to evaluate procedural success, long-term outcomes, and adverse events in real-world settings. This registry compiles data from patients receiving the Watchman device at 47 centers in 13 countries. A publication from the EWOLUTION registry in 2016 reported on 30-day outcomes of device implantation in 1021 patients.(25) The overall population had a risk of bleeding that was substantially higher than that for patients in the RCTs. Over 62% of patients included in the registry were deemed ineligible for anticoagulation by their physicians. Approximately one-third of patients had a history of major bleeding, and 40% had HAS-BLED scores of 3 or greater, indicating moderate-to-high risk of bleeding. Procedural success was achieved in 98.5% of patients, and 99.3% of implants demonstrated no blood flow or minimal residual blood flow postprocedure. Serious adverse events due to the device or procedure occurred at an overall rate of 2.8% (95% CI, 1.9% to 4.0%) at 7 days and 3.6% (95% CI, 2.5% to 4.9%) at 30 days. The most common serious adverse event was major bleeding. Section Summary: Watchman Device The most relevant evidence on use of the Watchman device for LAAC in patients eligible for anticoagulation is from 2 industry-sponsored RCTs and a patient-level meta-analysis of those studies. This evidence suggests that the Watchman is associated with an increased periprocedural ischemic stroke risk, which is balanced against a decreased hemorrhagic stroke risk. While neither trial individually demonstrates definitive improvement in outcomes, the patient-level meta-analysis reported improvement for a range of clinical outcomes for patients treated with the Watchman device. The overall bleeding risk is greater for the Watchman device in the periprocedural period, but decreased after the initial periprocedural period. Lariat® Device A systematic review of published studies on the Lariat device was published in 2016.(26) No RCTs were identified. Five case series were selected, with a total of 309 patients (range, 4-154 patients) treated. The combined estimate of procedural success was 90.3%. One (0.3%) death was reported and 7 (2.3%) patients required urgent cardiac surgery. This reviewers also searched the MAUDE database for adverse events, and found 35 unique reports. Among the 35 reported complications, there were 5 deaths and 23 cases of emergency cardiac surgery. Individual case series continue to be published, including a large case series of 712 consecutive patients from 18 U.S. hospitals.(27) This series reported a procedural success rate of 95% and complete closure in 98%. There was 1 death and emergent cardiac surgery was required in 1.4%. Other individual case series are smaller, reporting success rates and complication rates in the same range.(28-32) Section Summary: Lariat Device There are no RCTs of the Lariat device for this indication. The available case series are not sufficient to determine treatment efficacy. Amplatzer® Cardiac Plug Device The available evidence on use of the Amplatzer device for left atrial occlusion consists of a number of case series. The largest series identified was by Nietlispach et al, which included 152 patients from a single institution in Europe.(33) Short-term complications occurred in 9.8% (15/152) of patients. Longer term adverse outcomes occurred in 7% of patients, including 2 strokes, 1 peripheral embolization, and 4 episodes of major bleeding. Device embolization occurred in 4.6% (7/152) of patients. Other reports of patients treated with the Amplatzer device include a series of 90 patients from Belgium,(34) 86 patients from Portugal,(35) 37 patients from Italy,(36) 35 patients from Spain,(37) 21 patients from Poland,(38) and 20 patients from China.(18) All series reported high procedural success rates, as well as various complications such as vascular complications, air embolism, esophageal injury, cardiac tamponade, and device embolization. Several other case series have reported on use of the Amplatzer device in patients with a contraindication to oral anticoagulation therapy. The largest reported outcomes, up to 4 years postprocedure, for 134 patients with nonvalvular AF and a long-term contraindication to oral anticoagulation treated with the Amplatzer device.(39) Patients had a median CHA2DS2-VASc score of 4 and were generally considered at high risk for bleeding complications. Procedural success occurred in 93.3%, and 3 major procedure-related complications (2 cases of cardiac tamponade, 1 case of pericardial effusion requiring drainage or surgery) occurred. Over a mean follow-up of 680 days, observed annual rates of ischemic strokes and any thromboembolic events were 0.8% and 2.5%, respectively. Other case series have been published in this population, ranging from 37 to 100 patients.(36,40-43) They also reported high success rates and low procedural complications. Section Summary: Amplatzer Cardiac Plug Device There are no RCTs of the Amplatzer device for this indication. The available case series are not sufficient to determine treatment efficacy. PLAATO Device The available evidence on outcomes following use of the PLAATO device for stroke prevention in AF comes from case series and cohort studies. Bayard et al reported on 180 patients with nonrheumatic AF and a contraindication to warfarin and who were treated with the Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device. (44) Placement was successful in 90% of patients. Two patients died within 24 hours of the procedure (1.1%), and 6 patients had cardiac tamponade (3.3%), with 2 requiring surgical drainage. Other case reports and small case series report complications, including multiple reports of thrombus formation at the site of device placement.(45,46) Section Summary: PLAATO Device There are no RCTs of the PLAATO device for this indication. The available case series are not sufficient to determine treatment efficacy. Ongoing and Unpublished Clinical Trials Some currently unpublished trials that might influence this policy are listed in Table 1. Table 1. Summary of Key Trials NCT No. Ongoing NCT02039167 NCT01182441 NCT02426944 Unpublished NCT01363895 NCT01628068 NCT01118299 Trial Name Planned Enrollment Completion Date WATCH Bleeding Episodes After Left Atrial Appendage Occlusion Versus Usual Care in Patients With Atrial FIBrillatIon and Severe to eNd-stage Chronic Kidney Disease (WatchAFIB in CKD) Evaluation of the Watchman LAA closure device in patients with atrial fibrillation versus long term warfarin therapy Left Atrial Appendage Closure vs Novel Anticoagulation Agents in Atrial Fibrillation 300 Jun 2017 475 Aug 2017 400 May 2020 Interventional Strategies in Treatment of Atrial Fibrillation: Percutaneous Closure of the Left Atrial Appendage Versus Catheter Ablation Efficacy of Left Atrial Appendage Closure After Gastrointestinal Bleeding AMPLATZER Cardiac Plug Clinical Trial 120 Nov 2013 120 Jul 2014 3000 Not approved/ cleared NCT: national clinical trial. Summary of Evidence For individuals who have atrial fibrillation (AF) who are at increased risk for embolic stroke who receive the Watchman percutaneous left atrial appendage closure (LAAC) device, the evidence includes 2 randomized controlled trials (RCTs) and meta-analyses of these trials. Relevant outcomes are overall survival, morbid events, and treatment-related morbidity. The most relevant evidence comes from 2 industry-sponsored RCTs that compared the Watchman device with anticoagulation. One trial reported noninferiority on a composite outcome of stroke, cardiovascular/unexplained death, or systemic embolism after 2 years of follow-up, with continued benefits with the Watchman device after 4 years of follow-up. The second trial did not demonstrate noninferiority for the same composite outcome, but did demonstrate noninferiority of the Watchman device to warfarin for late ischemic stroke and systemic embolization. Patient-level meta-analyses of the 2 trials reported that the Watchman device is noninferior to warfarin on the composite outcome of stroke, systemic embolism, and cardiovascular death. Also, the Watchman was associated with a higher periprocedural risk of bleeding and ischemic stroke but a lower risk of hemorrhagic stroke over the long term. The published evidence indicates that the Watchman device is efficacious in preventing stroke for patients with AF who are at increased risk for embolic stroke. When it is determined on an individualized basis that the long-term risk of systemic anticoagulation exceeds the procedural risk of device implantation, the net health outcome will be improved. The evidence is sufficient to determine qualitatively that the technology results in a meaningful improvement in the net health outcome. For individuals who have AF who are at increased risk for embolic stroke who receive a percutaneous LAAC device other than the Watchman device (e.g, the Lariat, Amplatzer, and PLAATO devices), the evidence includes uncontrolled case series. Relevant outcomes are overall survival, morbid events, and treatment-related morbidity. Case series of these devices have reported high procedural success, but also numerous complications. In addition, these devices do not have the U.S. Food and Drug Administration approval for LAAC. The evidence is insufficient to determine the effects of the technology on health outcomes. Clinical Input Received From Physician Specialty Societies and Academic Medical Centers While the various physician specialty societies and academic medical centers may collaborate with and make recommendations during this process, through the provision of appropriate reviewers, input received does not represent an endorsement or position statement by the physician specialty societies or academic medical centers, unless otherwise noted. 2015 Input In response to requests, input was received from 1 physician specialty society (2 responses) and 4 academic medical centers, one of which provided 4 responses, for a total of 8 responses, while this policy was under review in 2015. The input generally supported the use of an FDA-approved LAA closure device for patients with an increased risk of stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc score and systemic anticoagulation therapy is recommended but the long-term risks of systemic anticoagulation outweigh the risks of the device implantation. Practice Guidelines and Position Statements American College of Cardiology, Heart Rhythm Society, et al. In 2015, the American College of Cardiology (ACC), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography and Interventions published an overview of the integration of percutaneous LAA closure devices into the clinical practice of patients with AF.(4) The overview was organized around questions related to the sites of care delivery for LAA closure devices, training for proceduralists, necessary follow-up data collection, identification of appropriate patient cohorts, and reimbursement. The statement provides general guidelines for facility and operator requirements, including the presence of a multidisciplinary heart team, for centers performing percutaneous LAA closures. The statement does not provide specific recommendations about the indications and patient populations appropriate for percutaneous LAA closure. American College of Cardiology, American Heart Association, et al. In 2014, the ACC, American Heart Association, and HRS issued guidelines on the management of patients with AF. (3) These guidelines recommend that surgical excision of the LAA may be considered in patients undergoing cardiac surgery (class IIB recommendation; level of evidence: C), but make no specific recommendations regarding percutaneous LAA closure. American College of Chest Physicians In 2012, the American College of Chest Physicians published evidence-based clinical best practice guidelines on the use of antithrombotic therapy for prevention of stroke in AF.(1) In relation to the use of LAA closure devices, the guidelines state: “At this time, we make no formal recommendations regarding LAA closure devices, pending more definitive research in this field.” U.S. Preventive Services Task Force Recommendations Not applicable. Medicare National Coverage There is no national coverage determination (NCD). In the absence of an NCD, coverage decisions are left to the discretion of local Medicare carriers. References [TOP] 1. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. Feb 2012;141(2 Suppl):e531S-575S. PMID 22315271 2. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. Nov 2010;138(5):10931100. PMID 20299623 3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. Dec 2 2014;64(21):e176. PMID 24685669 4. Administration FaD. Approval Letter: WATCHMAN LAA Closure Technology. 2015; http://www.accessdata.fda.gov/cdrh_docs/pdf13/p130013a.pdf. Accessed June, 16, 2016. 5. Blue Cross and Blue Shield Association Technology Evaluation Center (TEC). Percutaneous left atrial appendage closure therapy for prevention of stroke. TEC Assessments 2014;29; Tab 5. 6. Bode WD, Patel N, Gehi AK. Left atrial appendage occlusion for prevention of stroke in nonvalvular atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. Jun 2015;43(1):79-89. PMID 25711953 7. Briceno DF, Villablanca P, Cyrille N, et al. Left Atrial Appendage Occlusion Device and Novel Oral Anticoagulants Versus Warfarin for Stroke Prevention in Nonvalvular Atrial Fibrillation: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ Arrhythm Electrophysiol. Oct 2015;8(5):1057-1064. PMID 26226997 8. Holmes DR, Jr., Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. Jun 23 2015;65(24):2614-2623. PMID 26088300 9. Li X, Wen SN, Li SN, et al. Over 1-year efficacy and safety of left atrial appendage occlusion versus novel oral anticoagulants for stroke prevention in atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials and observational studies. Heart Rhythm. Dec 24 2015. PMID 26724488 10. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. May 19 2015;313(19):1950-1962. PMID 25988464 11. Price MJ, Reddy VY, Valderrabano M, et al. Bleeding outcomes after left atrial appendage closure compared with long-term warfarin: a pooled, patient-level analysis of the WATCHMAN randomized trial experience. JACC Cardiovasc Interv. Dec 28 2015;8(15):1925-1932. PMID 26627989 12. Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. Aug 15 2009;374(9689):534-542. PMID 19683639 13. Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) trial. Circulation. Feb 12 2013;127(6):720-729. PMID 23325525 14. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. Nov 19 2014;312(19):1988-1998. PMID 25399274 15. Alli O, Doshi S, Kar S, et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. Apr 30 2013;61(17):1790-1798. PMID 23500276 16. FDA. 2013 Meeting Materials of the Circulatory System Devices Panel. 2013; http://www.fda.gov/advisorycommittees/committeesmeetingmaterials/medicaldevices/medicaldevicesadvi sorycommittee/circulatorysystemdevicespanel/ucm342357.htm Accessed December 2016. 17. Holmes DR, Jr., Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. Jul 8 2014;64(1):1-12. PMID 24998121 18. Lam YY, Yip GW, Yu CM, et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. Apr 1 2012;79(5):794-800. PMID 21542102 19. Montenegro MJ, Quintella EF, Damonte A, et al. Percutaneous occlusion of left atrial appendage with the Amplatzer Cardiac PlugTM in atrial fibrillation. Arq Bras Cardiol. Jan 31 2012;98(2):143-150. PMID 22286325 20. Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. Apr 1 2011;77(5):700-706. PMID 20824765 21. Reddy VY, Holmes D, Doshi SK, et al. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. Feb 1 2011;123(4):417-424. PMID 21242484 22. Swaans MJ, Post MC, Rensing BJ, et al. Percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation. Neth Heart J. Jan 10 2012;20(4):161-166. PMID 22231152 23. Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulationthe ASAP Study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61(25):2551-2556. PMID 24. Chun KR, Bordignon S, Urban V, et al. Left atrial appendage closure followed by 6 weeks of antithrombotic therapy: a prospective single-center experience. Heart Rhythm. Dec 2013;10(12):17921799. PMID 23973952 25. Boersma LV, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. Jan 27 2016. PMID 26822918 26. Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and Procedural Success of Left Atrial Appendage Exclusion With the Lariat Device: A Systematic Review of Published Reports and Analytic Review of the FDA MAUDE Database. JAMA Intern Med. Jul 2015;175(7):1104-1109. PMID 25938303 27. Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: Results from a US multicenter evaluation. Heart Rhythm. May 2016;13(5):1030-1036. PMID 26872554 28. Price MJ, Gibson DN, Yakubov SJ, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol. Aug 12 2014;64(6):565-572. PMID 25104525 29. Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. Jul 9 2013;62(2):108-118. PMID 23062528 30. Bartus K, Bednarek J, Myc J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. Feb 2011;8(2):188-193. PMID 21050893 31. Stone D, Byrne T, Pershad A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation. Catheter Cardiovasc Interv. Jul 2015;86(1):121-127. PMID 23765504 32. Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol. Jan 9 2013;111(6):869-873. PMID 23312129 33. Nietlispach F, Gloekler S, Krause R, et al. Amplatzer left atrial appendage occlusion: Single center 10year experience. Catheter Cardiovasc Interv. Feb 14 2013;82(2):283-289. PMID 23412815 34. Kefer J, Vermeersch P, Budts W, et al. Transcatheter left atrial appendage closure for stroke prevention in atrial fibrillation with Amplatzer cardiac plug: the Belgian Registry. Acta Cardiol. Dec 2013;68(6):551558. PMID 24579432 35. Guerios EE, Schmid M, Gloekler S, et al. Left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation. Arq Bras Cardiol. Jun 2012;98(6):528-536. PMID 22584492 36. Danna P, Proietti R, Sagone A, et al. Does left atrial appendage closure with a cardiac plug system reduce the stroke risk in nonvalvular atrial fibrillation patients? A single-center case series. Pacing Clin Electrophysiol. Mar 2013;36(3):347-353. PMID 23252940 37. Lopez-Minguez JR, Eldoayen-Gragera J, Gonzalez-Fernandez R, et al. Immediate and one-year results in 35 consecutive patients after closure of left atrial appendage with the Amplatzer Cardiac Plug. Rev Esp Cardiol. Feb 2013;66(2):90-97. PMID 22939161 38. Streb W, Szymala M, Kukulski T, et al. Percutaneous closure of the left atrial appendage using the Amplatzer Cardiac Plug in patients with atrial fibrillation: evaluation of safety and feasibility. Kardiol Pol. 2013;71(1):8-16. PMID 23348528 39. Santoro G, Meucci F, Stolcova M, et al. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. EuroIntervention. Feb 20 2016;11(10):1188-1194. PMID 25354761 40. Meerkin D, Butnaru A, Dratva D, et al. Early safety of the Amplatzer Cardiac Plug for left atrial appendage occlusion. Int J Cardiol. Oct 9 2013;168(4):3920-3925. PMID 23890886 41. Wiebe J, Bertog S, Franke J, et al. Safety of percutaneous left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation and contraindications to anticoagulation. Catheter Cardiovasc Interv. Apr 1 2014;83(5):796-802. PMID 24327462 42. Urena M, Rodes-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. Jul 9 2013;62(2):96-102. PMID 23665098 43. Gloekler S, Shakir S, Doblies J, et al. Early results of first versus second generation Amplatzer occluders for left atrial appendage closure in patients with atrial fibrillation. Clin Res Cardiol. Aug 2015;104(8):656665. PMID 25736061 44. Bayard YL, Omran H, Neuzil P, et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. Jun 2010;6(2):220-226. PMID 20562072 45. Cruz-Gonzalez I, Martin Moreiras J, Garcia E. Thrombus formation after left atrial appendage exclusion using an amplatzer cardiac plug device. Catheter Cardiovasc Interv. Nov 15 2011;78(6):970-973. PMID 21523900 46. Viles-Gonzalez JF, Reddy VY, Petru J, et al. Incomplete occlusion of the left atrial appendage with the percutaneous left atrial appendage transcatheter occlusion device is not associated with increased risk of stroke. J Interv Card Electrophysiol. Jan 2012;33(1):69-75. PMID 21947786 47. Masoudi FA, Calkins H, Kavinsky CJ, et al. 2015 ACC/HRS/SCAI left atrial appendage occlusion device societal overview. J Am Coll Cardiol. Sep 29 2015;66(13):1497-1513. PMID 26133570 48. Blue Cross Blue Shield Association. Left-Atrial Appendage Closure Devices for Stroke Prevention in Atrial Fibrillation. Medical Policy Reference Manual, Policy 2.02.26, 2016. Appendix [TOP] N/A History [TOP] Date 06/13/11 12/29/11 05/22/12 09/17/12 05/28/13 09/23/14 12/08/15 07/12/16 01/01/17 Reason Add to Cardiology Section - New medical policy created with literature search; procedure considered investigational. Code 0281T added. Replace policy. Policy updated with literature review, references 2-4, 6-9, 11, 12 added. Policy title and policy statements revised to include percutaneous – no other change to policy statement. Update Coding Section – ICD-10 codes are now effective 10/01/2014. Replace policy. Policy updated with literature review through January 2013, references 2, 12-20 added. Policy statement unchanged. Annual Review. Policy updated with literature review through June 5, 2014. References 1-3, 12-14, 17, 25-29, 31-32, and 34 added. Policy statement unchanged. Annual Review. Policy updated with literature review through May 29, 2015; references 2-3, 6, 9, 12, 21, 33, and 43 added; clinical input reviewed. An FDA-approved left atrial appendage closure device is considered medically necessary with conditions. Annual Review. Policy updated with literature review through April 30, 2016; references 6-7, 9-11, and 25-27 added. Policy statements unchanged. Coding update, added new code 33340 effective 1/1/17. Disclaimer: This medical policy is a guide in evaluating the medical necessity of a particular service or treatment. The Company adopts policies after careful review of published peer-reviewed scientific literature, national guidelines and local standards of practice. Since medical technology is constantly changing, the Company reserves the right to review and update policies as appropriate. Member contracts differ in their benefits. Always consult the member benefit booklet or contact a member service representative to determine coverage for a specific medical service or supply. CPT codes, descriptions and materials are copyrighted by the American Medical Association (AMA). ©2017 Premera All Rights Reserved. Discrimination is Against the Law Premera Blue Cross complies with applicable Federal civil rights laws and does not discriminate on the basis of race, color, national origin, age, disability, or sex. Premera does not exclude people or treat them differently because of race, color, national origin, age, disability or sex. Premera: • Provides free aids and services to people with disabilities to communicate effectively with us, such as: • Qualified sign language interpreters • Written information in other formats (large print, audio, accessible electronic formats, other formats) • Provides free language services to people whose primary language is not English, such as: • Qualified interpreters • Information written in other languages If you need these services, contact the Civil Rights Coordinator. If you believe that Premera has failed to provide these services or discriminated in another way on the basis of race, color, national origin, age, disability, or sex, you can file a grievance with: Civil Rights Coordinator - Complaints and Appeals PO Box 91102, Seattle, WA 98111 Toll free 855-332-4535, Fax 425-918-5592, TTY 800-842-5357 Email [email protected] You can file a grievance in person or by mail, fax, or email. If you need help filing a grievance, the Civil Rights Coordinator is available to help you. You can also file a civil rights complaint with the U.S. Department of Health and Human Services, Office for Civil Rights, electronically through the Office for Civil Rights Complaint Portal, available at https://ocrportal.hhs.gov/ocr/portal/lobby.jsf, or by mail or phone at: U.S. Department of Health and Human Services 200 Independence Avenue SW, Room 509F, HHH Building Washington, D.C. 20201, 1-800-368-1019, 800-537-7697 (TDD) Complaint forms are available at http://www.hhs.gov/ocr/office/file/index.html. Getting Help in Other Languages This Notice has Important Information. This notice may have important information about your application or coverage through Premera Blue Cross. There may be key dates in this notice. You may need to take action by certain deadlines to keep your health coverage or help with costs. You have the right to get this information and help in your language at no cost. Call 800-722-1471 (TTY: 800-842-5357). አማሪኛ (Amharic): ይህ ማስታወቂያ አስፈላጊ መረጃ ይዟል። ይህ ማስታወቂያ ስለ ማመልከቻዎ ወይም የ Premera Blue Cross ሽፋን አስፈላጊ መረጃ ሊኖረው ይችላል። በዚህ ማስታወቂያ ውስጥ ቁልፍ ቀኖች ሊኖሩ ይችላሉ። የጤናን ሽፋንዎን ለመጠበቅና በአከፋፈል እርዳታ ለማግኘት በተውሰኑ የጊዜ ገደቦች እርምጃ መውሰድ ይገባዎት ይሆናል። ይህን መረጃ እንዲያገኙ እና ያለምንም ክፍያ በቋንቋዎ እርዳታ እንዲያገኙ መብት አለዎት።በስልክ ቁጥር 800-722-1471 (TTY: 800-842-5357) ይደውሉ። ( العربيةArabic): قد يحوي ھذا اإلشعار معلومات مھمة بخصوص طلبك أو.يحوي ھذا اإلشعار معلومات ھامة قد تكون ھناك تواريخ مھمة.Premera Blue Cross التغطية التي تريد الحصول عليھا من خالل وقد تحتاج التخاذ إجراء في تواريخ معينة للحفاظ على تغطيتك الصحية أو للمساعدة.في ھذا اإلشعار اتصل. يحق لك الحصول على ھذه المعلومات والمساعدة بلغتك دون تكبد أية تكلفة.في دفع التكاليف 800-722-1471 (TTY: 800-842-5357)بـ 中文 (Chinese): 本通知有重要的訊息。本通知可能有關於您透過 Premera Blue Cross 提交的 申請或保險的重要訊息。本通知內可能有重要日期。您可能需要在截止日期 之前採取行動,以保留您的健康保險或者費用補貼。您有權利免費以您的母 語得到本訊息和幫助。請撥電話 800-722-1471 (TTY: 800-842-5357)。 037338 (07-2016) Oromoo (Cushite): Beeksisni kun odeeffannoo barbaachisaa qaba. Beeksisti kun sagantaa yookan karaa Premera Blue Cross tiin tajaajila keessan ilaalchisee odeeffannoo barbaachisaa qabaachuu danda’a. Guyyaawwan murteessaa ta’an beeksisa kana keessatti ilaalaa. Tarii kaffaltiidhaan deeggaramuuf yookan tajaajila fayyaa keessaniif guyyaa dhumaa irratti wanti raawwattan jiraachuu danda’a. Kaffaltii irraa bilisa haala ta’een afaan keessaniin odeeffannoo argachuu fi deeggarsa argachuuf mirga ni qabaattu. Lakkoofsa bilbilaa 800-722-1471 (TTY: 800-842-5357) tii bilbilaa. Français (French): Cet avis a d'importantes informations. Cet avis peut avoir d'importantes informations sur votre demande ou la couverture par l'intermédiaire de Premera Blue Cross. Le présent avis peut contenir des dates clés. Vous devrez peut-être prendre des mesures par certains délais pour maintenir votre couverture de santé ou d'aide avec les coûts. Vous avez le droit d'obtenir cette information et de l’aide dans votre langue à aucun coût. Appelez le 800-722-1471 (TTY: 800-842-5357). Kreyòl ayisyen (Creole): Avi sila a gen Enfòmasyon Enpòtan ladann. Avi sila a kapab genyen enfòmasyon enpòtan konsènan aplikasyon w lan oswa konsènan kouvèti asirans lan atravè Premera Blue Cross. Kapab genyen dat ki enpòtan nan avi sila a. Ou ka gen pou pran kèk aksyon avan sèten dat limit pou ka kenbe kouvèti asirans sante w la oswa pou yo ka ede w avèk depans yo. Se dwa w pou resevwa enfòmasyon sa a ak asistans nan lang ou pale a, san ou pa gen pou peye pou sa. Rele nan 800-722-1471 (TTY: 800-842-5357). Deutsche (German): Diese Benachrichtigung enthält wichtige Informationen. Diese Benachrichtigung enthält unter Umständen wichtige Informationen bezüglich Ihres Antrags auf Krankenversicherungsschutz durch Premera Blue Cross. Suchen Sie nach eventuellen wichtigen Terminen in dieser Benachrichtigung. Sie könnten bis zu bestimmten Stichtagen handeln müssen, um Ihren Krankenversicherungsschutz oder Hilfe mit den Kosten zu behalten. Sie haben das Recht, kostenlose Hilfe und Informationen in Ihrer Sprache zu erhalten. Rufen Sie an unter 800-722-1471 (TTY: 800-842-5357). Hmoob (Hmong): Tsab ntawv tshaj xo no muaj cov ntshiab lus tseem ceeb. Tej zaum tsab ntawv tshaj xo no muaj cov ntsiab lus tseem ceeb txog koj daim ntawv thov kev pab los yog koj qhov kev pab cuam los ntawm Premera Blue Cross. Tej zaum muaj cov hnub tseem ceeb uas sau rau hauv daim ntawv no. Tej zaum koj kuj yuav tau ua qee yam uas peb kom koj ua tsis pub dhau cov caij nyoog uas teev tseg rau hauv daim ntawv no mas koj thiaj yuav tau txais kev pab cuam kho mob los yog kev pab them tej nqi kho mob ntawd. Koj muaj cai kom lawv muab cov ntshiab lus no uas tau muab sau ua koj hom lus pub dawb rau koj. Hu rau 800-722-1471 (TTY: 800-842-5357). Iloko (Ilocano): Daytoy a Pakdaar ket naglaon iti Napateg nga Impormasion. Daytoy a pakdaar mabalin nga adda ket naglaon iti napateg nga impormasion maipanggep iti apliksayonyo wenno coverage babaen iti Premera Blue Cross. Daytoy ket mabalin dagiti importante a petsa iti daytoy a pakdaar. Mabalin nga adda rumbeng nga aramidenyo nga addang sakbay dagiti partikular a naituding nga aldaw tapno mapagtalinaedyo ti coverage ti salun-atyo wenno tulong kadagiti gastos. Adda karbenganyo a mangala iti daytoy nga impormasion ken tulong iti bukodyo a pagsasao nga awan ti bayadanyo. Tumawag iti numero nga 800-722-1471 (TTY: 800-842-5357). Italiano (Italian): Questo avviso contiene informazioni importanti. Questo avviso può contenere informazioni importanti sulla tua domanda o copertura attraverso Premera Blue Cross. Potrebbero esserci date chiave in questo avviso. Potrebbe essere necessario un tuo intervento entro una scadenza determinata per consentirti di mantenere la tua copertura o sovvenzione. Hai il diritto di ottenere queste informazioni e assistenza nella tua lingua gratuitamente. Chiama 800-722-1471 (TTY: 800-842-5357). 日本語 (Japanese): この通知には重要な情報が含まれています。この通知には、Premera Blue Cross の申請または補償範囲に関する重要な情報が含まれている場合があ ります。この通知に記載されている可能性がある重要な日付をご確認くだ さい。健康保険や有料サポートを維持するには、特定の期日までに行動を 取らなければならない場合があります。ご希望の言語による情報とサポー トが無料で提供されます。800-722-1471 (TTY: 800-842-5357)までお電話 ください。 Română (Romanian): Prezenta notificare conține informații importante. Această notificare poate conține informații importante privind cererea sau acoperirea asigurării dumneavoastre de sănătate prin Premera Blue Cross. Pot exista date cheie în această notificare. Este posibil să fie nevoie să acționați până la anumite termene limită pentru a vă menține acoperirea asigurării de sănătate sau asistența privitoare la costuri. Aveți dreptul de a obține gratuit aceste informații și ajutor în limba dumneavoastră. Sunați la 800-722-1471 (TTY: 800-842-5357). 한국어 (Korean): 본 통지서에는 중요한 정보가 들어 있습니다. 즉 이 통지서는 귀하의 신청에 관하여 그리고 Premera Blue Cross 를 통한 커버리지에 관한 정보를 포함하고 있을 수 있습니다. 본 통지서에는 핵심이 되는 날짜들이 있을 수 있습니다. 귀하는 귀하의 건강 커버리지를 계속 유지하거나 비용을 절감하기 위해서 일정한 마감일까지 조치를 취해야 할 필요가 있을 수 있습니다. 귀하는 이러한 정보와 도움을 귀하의 언어로 비용 부담없이 얻을 수 있는 권리가 있습니다. 800-722-1471 (TTY: 800-842-5357) 로 전화하십시오. Pусский (Russian): Настоящее уведомление содержит важную информацию. Это уведомление может содержать важную информацию о вашем заявлении или страховом покрытии через Premera Blue Cross. В настоящем уведомлении могут быть указаны ключевые даты. Вам, возможно, потребуется принять меры к определенным предельным срокам для сохранения страхового покрытия или помощи с расходами. Вы имеете право на бесплатное получение этой информации и помощь на вашем языке. Звоните по телефону 800-722-1471 (TTY: 800-842-5357). ລາວ (Lao): ແຈ້ ງການນ້ີ ມີຂ້ໍ ມູ ນສໍາຄັ ນ. ແຈ້ ງການນ້ີ ອາດຈະມີຂ້ໍ ມູ ນສໍາຄັ ນກ່ ຽວກັ ບຄໍາຮ້ ອງສະ ໝັ ກ ຫື ຼ ຄວາມຄຸ້ ມຄອງປະກັ ນໄພຂອງທ່ ານຜ່ ານ Premera Blue Cross. ອາດຈະມີ ວັ ນທີສໍາຄັ ນໃນແຈ້ ງການນີ້. ທ່ ານອາດຈະຈໍາເປັນຕ້ ອງດໍາເນີນການຕາມກໍານົ ດ ເວລາສະເພາະເພື່ອຮັ ກສາຄວາມຄຸ້ ມຄອງປະກັ ນສຸ ຂະພາບ ຫື ຼ ຄວາມຊ່ ວຍເຫື ຼ ອເລື່ອງ ຄ່ າໃຊ້ ຈ່ າຍຂອງທ່ ານໄວ້ . ທ່ ານມີສິດໄດ້ ຮັ ບຂ້ໍ ມູ ນນ້ີ ແລະ ຄວາມຊ່ ວຍເຫື ຼ ອເປັນພາສາ ຂອງທ່ ານໂດຍບໍ່ເສຍຄ່ າ. ໃຫ້ ໂທຫາ 800-722-1471 (TTY: 800-842-5357). ភាសាែខម រ (Khmer): េសចកត ីជូនដំណឹងេនះមានព័ត៌មានយា៉ងសំខាន់។ េសចកត ីជូនដំណឹងេនះរបែហល ជាមានព័ត៌មានយា៉ងសំខាន់អំពីទរមង់ែបបបទ ឬការរា៉ប់រងរបស់អនកតាមរយៈ Premera Blue Cross ។ របែហលជាមាន កាលបរ ិេចឆ ទសំខាន់េនៅកនុងេសចកត ីជូន ដំណឹងេនះ។ អន ករបែហលជារតូវការបេញច ញសមតថ ភាព ដល់កំណត់ៃថង ជាក់ចបាស់ នានា េដើមបីនឹងរកសាទុកការធានារា៉ប់រងសុខភាពរបស់អនក ឬរបាក់ជំនួយេចញៃថល ។ អន កមានសិទធិទទួ លព័ត៌មានេនះ និងជំនួយេនៅកនុងភាសារបស់អនកេដាយមិនអស លុយេឡើយ។ សូ មទូ រស័ពទ 800-722-1471 (TTY: 800-842-5357)។ ਪੰ ਜਾਬੀ (Punjabi): ਇਸ ਨੋਿਟਸ ਿਵਚ ਖਾਸ ਜਾਣਕਾਰੀ ਹੈ. ਇਸ ਨੋਿਟਸ ਿਵਚ Premera Blue Cross ਵਲ ਤੁਹਾਡੀ ਕਵਰੇਜ ਅਤੇ ਅਰਜੀ ਬਾਰੇ ਮਹੱ ਤਵਪੂਰਨ ਜਾਣਕਾਰੀ ਹੋ ਸਕਦੀ ਹੈ . ਇਸ ਨੋਿਜਸ ਜਵਚ ਖਾਸ ਤਾਰੀਖਾ ਹੋ ਸਕਦੀਆਂ ਹਨ. ਜੇਕਰ ਤੁਸੀ ਜਸਹਤ ਕਵਰੇਜ ਿਰੱ ਖਣੀ ਹੋਵੇ ਜਾ ਓਸ ਦੀ ਲਾਗਤ ਜਿਵੱ ਚ ਮਦਦ ਦੇ ਇਛੁੱ ਕ ਹੋ ਤਾਂ ਤੁਹਾਨੂੰ ਅੰ ਤਮ ਤਾਰੀਖ਼ ਤ ਪਿਹਲਾਂ ਕੁੱ ਝ ਖਾਸ ਕਦਮ ਚੁੱ ਕਣ ਦੀ ਲੋ ੜ ਹੋ ਸਕਦੀ ਹੈ ,ਤੁਹਾਨੂੰ ਮੁਫ਼ਤ ਿਵੱ ਚ ਤੇ ਆਪਣੀ ਭਾਸ਼ਾ ਿਵੱ ਚ ਜਾਣਕਾਰੀ ਅਤੇ ਮਦਦ ਪ੍ਰਾਪਤ ਕਰਨ ਦਾ ਅਿਧਕਾਰ ਹੈ ,ਕਾਲ 800-722-1471 (TTY: 800-842-5357). ( فارسیFarsi): اين اعالميه ممکن است حاوی اطالعات مھم درباره فرم. اين اعالميه حاوی اطالعات مھم ميباشد به تاريخ ھای مھم در. باشدPremera Blue Cross تقاضا و يا پوشش بيمه ای شما از طريق شما ممکن است برای حقظ پوشش بيمه تان يا کمک در پرداخت ھزينه. اين اعالميه توجه نماييد شما حق. به تاريخ ھای مشخصی برای انجام کارھای خاصی احتياج داشته باشيد،ھای درمانی تان برای کسب.اين را داريد که اين اطالعات و کمک را به زبان خود به طور رايگان دريافت نماييد ( تماس800-842-5357 تماس باشمارهTTY )کاربران800-722-1471 اطالعات با شماره .برقرار نماييد Polskie (Polish): To ogłoszenie może zawierać ważne informacje. To ogłoszenie może zawierać ważne informacje odnośnie Państwa wniosku lub zakresu świadczeń poprzez Premera Blue Cross. Prosimy zwrócic uwagę na kluczowe daty, które mogą być zawarte w tym ogłoszeniu aby nie przekroczyć terminów w przypadku utrzymania polisy ubezpieczeniowej lub pomocy związanej z kosztami. Macie Państwo prawo do bezpłatnej informacji we własnym języku. Zadzwońcie pod 800-722-1471 (TTY: 800-842-5357). Português (Portuguese): Este aviso contém informações importantes. Este aviso poderá conter informações importantes a respeito de sua aplicação ou cobertura por meio do Premera Blue Cross. Poderão existir datas importantes neste aviso. Talvez seja necessário que você tome providências dentro de determinados prazos para manter sua cobertura de saúde ou ajuda de custos. Você tem o direito de obter esta informação e ajuda em seu idioma e sem custos. Ligue para 800-722-1471 (TTY: 800-842-5357). Fa’asamoa (Samoan): Atonu ua iai i lenei fa’asilasilaga ni fa’amatalaga e sili ona taua e tatau ona e malamalama i ai. O lenei fa’asilasilaga o se fesoasoani e fa’amatala atili i ai i le tulaga o le polokalame, Premera Blue Cross, ua e tau fia maua atu i ai. Fa’amolemole, ia e iloilo fa’alelei i aso fa’apitoa olo’o iai i lenei fa’asilasilaga taua. Masalo o le’a iai ni feau e tatau ona e faia ao le’i aulia le aso ua ta’ua i lenei fa’asilasilaga ina ia e iai pea ma maua fesoasoani mai ai i le polokalame a le Malo olo’o e iai i ai. Olo’o iai iate oe le aia tatau e maua atu i lenei fa’asilasilaga ma lenei fa’matalaga i legagana e te malamalama i ai aunoa ma se togiga tupe. Vili atu i le telefoni 800-722-1471 (TTY: 800-842-5357). Español (Spanish): Este Aviso contiene información importante. Es posible que este aviso contenga información importante acerca de su solicitud o cobertura a través de Premera Blue Cross. Es posible que haya fechas clave en este aviso. Es posible que deba tomar alguna medida antes de determinadas fechas para mantener su cobertura médica o ayuda con los costos. Usted tiene derecho a recibir esta información y ayuda en su idioma sin costo alguno. Llame al 800-722-1471 (TTY: 800-842-5357). Tagalog (Tagalog): Ang Paunawa na ito ay naglalaman ng mahalagang impormasyon. Ang paunawa na ito ay maaaring naglalaman ng mahalagang impormasyon tungkol sa iyong aplikasyon o pagsakop sa pamamagitan ng Premera Blue Cross. Maaaring may mga mahalagang petsa dito sa paunawa. Maaring mangailangan ka na magsagawa ng hakbang sa ilang mga itinakdang panahon upang mapanatili ang iyong pagsakop sa kalusugan o tulong na walang gastos. May karapatan ka na makakuha ng ganitong impormasyon at tulong sa iyong wika ng walang gastos. Tumawag sa 800-722-1471 (TTY: 800-842-5357). ไทย (Thai): ประกาศนี ้มีข้อมูลสําคัญ ประกาศนี ้อาจมีข้อมูลที่สําคัญเกี่ยวกับการการสมัครหรื อขอบเขตประกัน สุขภาพของคุณผ่าน Premera Blue Cross และอาจมีกําหนดการในประกาศนี ้ คุณอาจจะต้ อง ดําเนินการภายในกําหนดระยะเวลาที่แน่นอนเพื่อจะรักษาการประกันสุขภาพของคุณหรื อการช่วยเหลือที่ มีค่าใช้ จ่าย คุณมีสิทธิที่จะได้ รับข้ อมูลและความช่วยเหลือนี ้ในภาษาของคุณโดยไม่มีค่าใช้ จ่าย โทร 800-722-1471 (TTY: 800-842-5357) Український (Ukrainian): Це повідомлення містить важливу інформацію. Це повідомлення може містити важливу інформацію про Ваше звернення щодо страхувального покриття через Premera Blue Cross. Зверніть увагу на ключові дати, які можуть бути вказані у цьому повідомленні. Існує імовірність того, що Вам треба буде здійснити певні кроки у конкретні кінцеві строки для того, щоб зберегти Ваше медичне страхування або отримати фінансову допомогу. У Вас є право на отримання цієї інформації та допомоги безкоштовно на Вашій рідній мові. Дзвоніть за номером телефону 800-722-1471 (TTY: 800-842-5357). Tiếng Việt (Vietnamese): Thông báo này cung cấp thông tin quan trọng. Thông báo này có thông tin quan trọng về đơn xin tham gia hoặc hợp đồng bảo hiểm của quý vị qua chương trình Premera Blue Cross. Xin xem ngày quan trọng trong thông báo này. Quý vị có thể phải thực hiện theo thông báo đúng trong thời hạn để duy trì bảo hiểm sức khỏe hoặc được trợ giúp thêm về chi phí. Quý vị có quyền được biết thông tin này và được trợ giúp bằng ngôn ngữ của mình miễn phí. Xin gọi số 800-722-1471 (TTY: 800-842-5357).