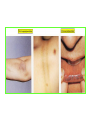
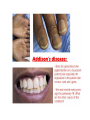
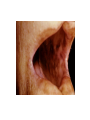
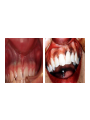
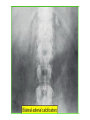
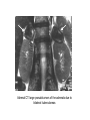
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Adrenal failure
Growth hormone therapy wikipedia , lookup
Hypothalamic–pituitary–adrenal axis wikipedia , lookup
Signs and symptoms of Graves' disease wikipedia , lookup
Hypopituitarism wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency wikipedia , lookup
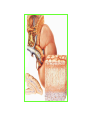
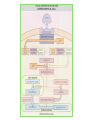
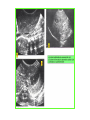
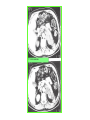
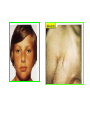
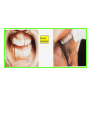
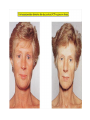
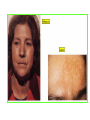
THE ADRENALS ADRENAL CORTEX: is formed in the weeks V-VI: Cells are derived from the cells derived from genital crest which will form sterodegenetic cell from adrenal glands and gonads. They develop under the control of genes SF- 1, DAX ADRENAL MEDULLA: is of neuroectodermic origin Fetal adrenal glands are formed of 3 zones: “definitive” under the capsulla (it produces mineralocorticoids ) of transition ( glucocorticoids) fetal zone has the most important area during fetal life and produces androgens - DHEA-S which are transformed within e placenta into estrogens zona glomerulosa and fasciculata are completely formed at 3 years, and zona reticularis at 15 years Zona reticularis Zona fasciculata Zona glomerulosa STERODOGENESIS IN THE ADRENAL CORTEX • Pattern of steroidogenesis within the adrenal cortex Glucocorticoid synthesis is produced into zona fasciculata Cholesterol is converted into pregnenolone due to an enzyme that produces the clivage of lateral chain of cholesterol and StAR – steroidogenetic acute regulatory protein 1.Pregnenolone is transformed into 17 hydroxi-pregnenolone due to enzyme 17 hydroxilase (C17) 2. 17 hydroxi-pregnenolone in transformed into 17 hyroxiprogesterone due to 3β hydroxysteroid dehydrogenase 3. 17 hyroxiprogesterone is transformed into 11 deoxy-cortisole by 21 hydroxilase 4.11 deoxy-cortisole is converted into cortisole by 11 β hydroxilase Mineralocorticoid biosynthesis 1. Cholesterol is converted into pregnenolone due to an enzyme that produces the clivage of lateral chain of cholesterol and StAR – steroidogenetic acute regulatory protein 2. Pregnenolone is transformed into progesterone due to enzyme 3β hydroxysteroid dehydrogenase 3. Progesterone is converted into deoxycorticosterone under the action of 21 hydroxilase 4. Deoxycorticosterone is convertes into corticosterone due to 11 β hydroxilase 5. Corticosterone is transformed into 18 hydroxi-corticosterone due to 18 hyroxilase (corticosterone methyl oxidase I) 6. 18 hydroxi-corticosterone is converted into aldosterone due to 18 hydrogenase (corticosterone methil oxidase II) Steroid Production rate Carrier protein Cortisole 8-25 mg/24 h Corticosteroidbinding 3-10 % Globulin (CBG) Transcortin – 90 % albumin 20-140 (F) 40-180 (M) Aldosterone DOC 0.15 mg/24 ore 0,6 mg /14 ore Transcortin: 40 % aldo 20 % aldo and %24 % DOC DOC Albumin: 40 %aldo nd %DOC 0,15 - 0,17 0,15 – 0,17 DHEA DHEA-S Testosterone 0,7 mg /14 ore (F) SHBG 6-8 mg /24 ore TeBG 0,23 mg/24 ore (F) Sex hormone binding globulin Progesterone Estradiol (conversion % free fraction – Transcortin SHBG Plasma circulation of adrenal steroids Plasma concentration ng/ml 5,34 ± 1,57 1130 (F), 1260 (M) 0,48 ± 0,14 (F) 5,59 ± 1,51 (B) 11,8 ± 7 (F) 0,18 ± 0,1 (M Adrenal steroids metabolism LIVER CORTISOL E CO RTISOLE Kidney CO RTISOLE 3 HDH DIHY DROCO RTIZO LE T ETRAHY DROCO RTIZO L E CO RTIZO NE 11 HDH DIHY DROCO RTIZO NE T ETRAHY DROCO RTIZO N CO RTOLE Free ur inary cor tisole CO RTOLO NE GLICURONID TRANSFERASE GLIC URO NOC O NJUGATI URINE 17 OH -C S Steroid receptor 1 2 NH2 COOH Transactivational domain – regulates DNA tr nscr iption DNA -binding domain – activates the transcr iption DNA -binding domanin which is resposable for nuclear localisation of ster oid receptor and dymer asation Steroid receptors Receptor ligand Other ligands for the same receptor Receptor antagonist Main actions Area or sterod production Glucocorticoid corisolel Corticosterone Prednisone DXM RU 486 Intermediary metabolism and stress response Yona fasciculata and reticularis Mineralocorticoid Aldosterone cortisole DOC Spironolactone Na+ , K + Zona glomerulosa Androgen Dihydrotestoster one testosterone Spironolactone cyproterone Sexual and reprodcutive action in man Testisl adrenal Estrogens Estradiol Tamoxifen SERM Sexual and reproductive function in women Ovary, follicle, placenta Progesterone progesterone RU486 Sexual and reproductive function in women Ovary – yelow body and placenta Vitamin D 1,25 di hidroxi colecalciferole Calcium metabolism kidney Estrone Estriole GLUCOCORTICOD - ACTIONS Tissue and controlled metabolism Liver glucose metabolism Periferal glucose metabolism Actions and implications Increased neogluco geensis and l iv er response to othe stimulators (catheco lamines and glu cagon ) Increased substrate fo r neoglu cogenesis f rom amino acids rezultin g from protein catab olism and free fatty acids liberatio n Decreased glucose uptake by tissues w ith secondary insulin resistance Fat metabolism Increased ly polisis an free fatty acid liberation Ly pogenesis due to increase d apetite and high insuli n lev els Increased fat deposits in some areas of the body : face, thorax, abdomen, upper part of the bo dy Protein metabolism Increased protein metabol ism Inhibition of amino acid s uptake by tissues Glucocorticoid actions Tissue and metabolic control Growth Actions and implication S timulate grow th in phy siological con centratio ns G lucocortico id excess prod uces catabo lic effct s, decreses G h, IGF 1, and inhibits g row th G lucorticoid t reatment duri ng chil dhood may produce definitiv e grow th deficiency Erithropoesis stimulation Leucocytes S timulation of neuthrophi ls liberat ion from bone marrow Decreased circulat ing ly mphocy tes, macrophage migration and inflamation Incresed suscept ibility to infections i n endogenous o r exoge nous glucoco rtico id exce ss Immune system Inhibition of all facto r inv olved in immune response: macro phage migration, pros taglandin l iberation, ant igen presentat ion by macrophages, antibo dy production, interle ukine 1,2, liberation,, in gamma interferon,CSF , TNF, histamine, serotonine and bradikin ine liberation. GLUCOCORTICOID ACTIONS CONTROLLED TISSUE AND METABOLISM ACTIONS AND IMPLICATIONS CARDIOVASCULAR SYSTEM G lucocortico id excess may produce hy pertension KIDNEY Increased blood ci rculat ion in the kidney and glomerular filt ration A ntagonism w ith v asopressin CENTRAL NERVOUS SYSTEM AND BEHAVIOR G lucocortico id deficie ncy produces phy sical and psy chological tiredness G lucocortico id excess prod uces eupho ria follow ed by depression ENDOCRINE GLA NDS F eed-back control in the sy stem C RH/ACTH Thy roid: inhibit s TSH response to TRH and conv ersion of T4 in T3 G onads: inhibits l LH and F H response to LH -Rh decrease G H A drenal medulla: stimulates N E and E by activ ating thy rosinhy droxilaze OTHER ACTIONS Increased gastri c acid secretion w ith peptic ul cer du ring hig h dose glucoco rtico id treatment Increased intra ey e pressure Adrenal failure Adrenal insufficiency is a disorder first described by Thomas Addison in 1855, which is characterized by impaired adrenocortical function and decreased production of glucocorticoids, mineralocorticoids and/or adrenal androgens. Adrenal insufficiency can be caused by diseases affecting the adrenal cortex (primary), the pituitary gland and the secretion of adrenocorticotropic hormone (ACTH) (secondary) or the hypothalamus and the secretion of corticotropicreleasing hormone (CRH) (tertiary). Adrenal insuficiency TERTIARY - CRH SECONDARY - ACTH H h CHRONIC ACUTE PRIMARY :G-ALD-ANDRO CSR Primary – due to adrenal failure Secondary – due to ACTH deficiency Tertiary – due to CRH deficiency Prevalence: • 39-60 new cases in 1 million /year • 60-70 % of cases are diagnosed between 30 – 50 de years •Sex ratio : F/M = 1,25 /1 in tuberculosis si 2,6 - 3/1 in autoimmune adrenal failure Primary insufficiency adrenal Idiopathic (autoimmune) Tuberculosis other (Miller si Tyrel, Felig 1996) Autoimmune Tuberculosis Other Causes of adrenal failure between 1928-1938 17 79 4 67 – 70 15 15 Causes of adrnal failure between % 1962-1972 78 21 1 Causes of Primary Adrenal Insufficiency • The etiology of primary adrenal insufficiency has changed over time. Prior to 1920, the most common cause of primary adrenal insufficiency was tuberculosis, while since 1950, the majority of cases have been ascribed to autoimmune adrenalitis, either in isolation or in the context of complex polyglandular syndromes Causes of Primary Adrenal Insufficiency • Autoimmune adrenalitis : This condition is the result of an autoimmune process that destroys the adrenal cortex. Both humoral and cell-mediated immune mechanisms directed at the adrenal cortex are involved. The adrenals appear small and atrophic and the surrounding capsule is thickened. Antibodies that react with several steroidogenic enzymes, as well as all three zones of the adrenal cortex are detected in 60-75% of patients with autoimmune primary adrenal insufficiency, but only rarely in patients with other causes of adrenal insufficiency or normal subjects Causes of Primary Adrenal Insufficiency . Considerable progress has been made in identifying genetic factors that predispose to the development of autoimmune adrenal insufficiency . In addition to the well-known association of the HLA genotype DR3/4-DQB1*0302 with type 1 diabetes mellitus and adrenal insufficiency, a strong susceptibility for the latter was also found for the DR3-DQ2/DRB1*0404-DQ8 genotype, which predicted early onset of primary adrenal insufficiency Approximately 50% of patients with autoimmune adrenal insufficiency have one or more other autoimmune endocrine disorders, whereas patients with the more common autoimmune endocrine disorders, such as type 1 diabetes mellitus, chronic autoimmune thyroiditis, or Graves' disease, rarely develop adrenal insufficiency. The combination of autoimmune adrenal insufficiency with other autoimmune endocrine disorders is referred to as the polyglandular autoimmune syndromes type I and II Causes of Primary Adrenal Insufficiency • • • • • • • • • • • • • • • Primary Adrenal Insufficiency Autoimmune (polyglandular failure) Tuberculosis Sarcoidosis, amyloidosis, hemochromatosis Hemorrhage (meningococcemia, anticoagulants, trauma) Fungal infections Metastatic neoplasia/infiltration Congenital adrenal hyperplasia Congenital adrenal hypoplasia hypoplasia drenalis congenita Congenital unresponsiveness to ACTH (glucocorticoid deficiency, ACTH resistance) Adrenoleukodystrophy/Adrenomieloneuropathy Aquired immynodeficiency syndrome Bilateral adrenalectomy Steroid synthesis inhibitors (e.g., metyrapone, ketoconazole, aminoglutethimide) Adrenolytic agents (o,p’DDD, suramin) Glucocorticoid antagonists (RU 486) Causes of secondary and tertiary Adrenal Insufficiency • • • • • • • • • • • • • • • • Secondary and Tertiary Adrenal Insufficiency Following discontinuation of exogenous glucocorticoids or ACTH Following the cure of Cushing’s syndrome Pituitary and hypothalamic lesions Tumors Inflammation Infections Autoimmune lesions Granulomatios infilitration Trauma Congenital aplasia, hypoplasia, dysplasia, ectopy Pituitary-hypothalamic surgery Pituitary-hypothalamic radiation Pituitary-hypothalamic hemorrhage (apoplexy) Acquired isolated ACTH deficiency Familial corticosteroid-binding-globulin deficiency Causes of Primary Adrenal Insufficiency • • Infectious adrenalitis : Many infectious agents may attack the adrenal gland and result in adrenal insufficiency, including tuberculosis (tuberculous adrenalitis), disseminated fungal infections and HIV-associated infections, such as adrenalitis due to cytomegalovirus and mycobacterium avium complex . Hemorrhagic infarction: Bilateral adrenal infarction caused by hemorrhage or adrenal vein thrombosis may also lead to adrenal insufficiency . The diagnosis is usually made in critically ill patients in whom a computed tomography (CT) scan of the abdomen shows bilateral adrenal enlargement. Several coagulopathies and the heparin-induced thrombocytopenia syndrome have been associated with adrenal vein thrombosis and hemorrhage, while the primary antiphospholipid syndrome has been recognized as a major cause of adrenal hemorrhage . Adrenal hemorrhage has been mostly associated with meningococcemia (Waterhouse-Friderichsen syndrome) and Pseudomonas aeruginosa infection). Causes of Primary Adrenal Insufficiency • Adrenoleukodystrophy : This is an X-linked recessive disorder affecting 1 in 20.000 males . Adrenoleukodystrophy is characterized by spastic paralysis and adrenal insufficiency, usually beginning in infancy or childhood, and is caused by mutations in the ABCD1 gene, resulting in defective beta oxidation of very long chain fatty acids (VLCFAs) within peroxisomes. The abnormally high concentrations of VLCFAs in many organs, including the adrenal cortex, result in the clinical manifestations of this disorder. Causes of Primary Adrenal Insufficiency • • Drug-induced adrenal insufficiency : Drugs that may cause adrenal insufficiency by inhibiting cortisol biosynthesis, particularly in individuals with limited pituitary and/or adrenal reserve, include aminoglutethimide (antiepileptic), etomidate (anesthetic-sedative) , ketoconazole (antimycotic) and metyrapone. Drugs that accelerate the metabolism of cortisol and most synthetic glucocorticoids by inducing hepatic mixed-function oxygenase enzymes, such as phenytoin, barbiturates, and rifampicin can also cause adrenal insufficiency in patients with limited pituitary or adrenal reserve, as well as those who are on replacement therapy with glucocorticoids . Furthermore, some of novel tyrosine kinase-targeting drugs (e.g. sunitinib) have been shown in animal studies to cause adrenal dysfunction and Adrenal hemorrhage in the context of a sepsis, anticoagulant treatment . PATHOPHYSIOLOGY OF ADRENAL INSUFFICIENCY Pathophysiology of Primary Adrenal Insufficiency • In primary adrenal insufficiency, all the above mentioned causes result in gradual destruction of the adrenal cortex. However, the clinical manifestations of the condition appear when the loss of the adrenocortical tissue of both glands is higher than 90% . In the initial phase of chronic gradual destruction, the adrenal reserve is decreased and although the basal steroid secretion is normal, the secretion in response to stress is suboptimal. Consequently, any major or even minor stressor can precipitate an acute adrenal crisis. With further loss of adrenocortical tissue, even basal steroid secretion is decreased, leading to the clinical manifestations of the disease. Low plasma cortisol concentrations result in the increase of production and secretion of ACTH due to decreased negative feedback inhibition . The elevated plasma ACTH concentrations are responsible for the well-recognised hyperpigmentation observed in these patients. Pathophysiology of Secondary Adrenal Insufficiency • ACTH deficiency leads to decreased secretion of cortisol and adrenal androgens, while mineralocorticoid production remains normal. In the early stages, basal ACTH secretion is normal, while that of stress-induced is impaired . With further loss of basal ACTH secretion, there is atrophy of zonae fasciculata and reticularis of the adrenal cortex. Therefore, basal cortisol secretion is decreased but aldosterone secretion by the zona glomerulosa is preserved. Adrenal insufficiency signs and symptoms • Chronic Primary Adrenal Insufficiency: Patients with chronic primary adrenal insufficiency may have symptoms and signs of glucocorticoid, mineralocorticoid, and androgen deficiency. By contrast, patients with secondary or tertiary adrenal insufficiency usually have normal mineralocorticoid function. The onset of chronic adrenal insufficiency is often insidious and the diagnosis may be difficult in the early stages of the disease. Adrenal insuficiency signs and symptoms Clinical signs and symptoms occur when more than 90 % of adrenals is non functional. Glucocorticoid defficiency determines by feed-back an increased of pro-opio melanocortin and ACTH and MSH respectively . with skin pigmentation. Chronic adrenal insufficiency evolves in 4 stages: • aldosterone deficiency with increased plasma rennin activity. • subclinical cortisole deficiency with increased ACTH • low response of cortisole to SCTH stimulation test • Clinical signs and symptoms of the disease • Cortisole deficiency: hypoglycemia, lethargy, reducer apetite, anemia, depression. • Aldosterone deficiency: hyponatremia, reduced plasma volume , low blood pressure, reducered ability of kidney to respond to vasopressin and excrete free water. Adrenal insufficiency signs and symptoms • The most common clinical manifestations of chronic primary adrenal insufficiency include: • General malaise, fatigue, weakness, • anorexia, weight loss, • nausea, vomiting, abdominal pain or diarrhea, which may alternate with constipation, • hypotension, • electrolyte abnormalities (hyponatremia, hyperkalemia, metabolic acidosis), • hyperpigmentation, autoimmune manifestations (vitiligo), • decreased axillary and pubic hair, and loss of libido and amenorrhea in women Adrenal insufficiency signs and symptoms • Skin and mucosal pigmentation occurs on areas exposed to sun, exposed to pressure such as knuckles, toes, elbows and knees. Pigmentation of bucal mucosa and gums are preceded by generalized skin pigmentation. Increased pigmentation of palmar creases, nail beds, nipples, areolae, perivaginal and perianal mucosa is also found. Scars that have been formed after the onset of the diseas are also pigmented. Adrenal CT: large pseudotumors of the adrenals due to bilateral tuberculomas Symptoms Weakness, fatigue, Anorexia Gastro intestinal symptoms: nausea and vomiting, abdominal pain Salt craving Postural hypotension Muscle and joint pains 100 % 100 % 56-92 % 16-19 % 12 % 6-13 % Signs 100% 92-94 % 88-94 % 10-20 % 8% Weight loss Skin pigmentation Hypotension (TAS < 110 mm Hg) Vitiligo Hypoglicemie Laboratory data Hyponatremia Hyperkaliemie Hypercalcemie Hyperazotemie Anemia Eozinophilia 88 % 64 % 6% 55 % 40 % 17 % Adrenal Crisis • Adrenal Crisis: Adrenal crisis or acute adrenal insufficiency may complicate the course of chronic primary adrenal insufficiency, and may be precipitated by a serious infection, acute stress, bilateral adrenal infarction or hemorrhage. It is rare in patients with secondary or tertiary adrenal insufficiency. • The main clinical manifestation of adrenal crisis is shock, but patients may also have nonspecific symptoms, such as anorexia, nausea, vomiting, abdominal pain, weakness, fatigue, lethargy, confusion or coma. • Hypoglycemia is rare in acute adrenal insufficiency, but more common in secondary adrenal insufficiency. Hypoglycemia is a common manifestation in children and thin women with the disorder. • Hyperpigmentation due to chronic ACTH hypersecretion and weight loss are indicative of long-standing adrenal insufficiency, while additional symptoms and signs relating to the primary cause of adrenal insufficiency may also be present. Adrenal Crisis • The major factor precipitating an adrenal crisis is mineralocorticoid deficiency and the main clinical problem is hypotension. Adrenal crisis can occur in patients receiving appropriate doses of glucocorticoid if their mineralocorticoid requirements are not met whereas patients with secondary adrenal insufficiency and normal aldosterone secretion rarely present in adrenal crisis. However, glucocorticoid deficiency may also contribute to hypotension by decreasing vascular responsiveness to angiotensin II, norepinephrine and other vasoconstrictive hormones, reducing the synthesis of renin substrate, and increasing the production and effects of prostacyclin and other vasodilatory hormones Acute adrenal insufficiency during WaterhouseFrederikson) Syndrome = severe sepsis with adrenal insufficiency Secondary adrenal insufficiency • Secondary or Tertiary Adrenal Insufficiency: The clinical features of secondary or tertiary adrenal insufficiency are similar to those of primary adrenal insufficiency. However, hyperpigmentation is not present because ACTH secretion is not increased. • Also, given that the production of mineralococorticoids by the zona glomerulosa is mostly preserved, dehydration and hyperkalemia are not present, and hypotension is less prominent. • Hyponatremia and increased intravascular volume may be the result of “inappropriate” increase in vasopressin secretion. • Hypoglycemia is more common in secondary adrenal insufficiency possibly due to concomitant growth hormone insufficiency and in isolated ACTH deficiency. • Clinical manifestations of a pituitary or hypothalamic tumor, such as symptoms and signs of deficiency of other anterior pituitary hormones, headache or visual field defects, may also be present CHRONIC ADRENAL INSUFFICIENCY investigation flowchart CHINICAL SIGNS AND SYMPTOMS Hypo Na, hyperK, eosinophilia, acidosis, hypoglicemia, EKG Decreased plasma cortisole, urinary cortisole, 17KS, 17OH-CS 2 1 ACTH test 1 or 250 μg Cortisole not increased Cotisole increases Male >40 years Female, ACTH dosage:increased Adrenal calcifications Other autoimmune disease Megative metopirone test Adrenal TB Antibodies against 21 OH, 11 OH Autoimmune 3 Imagery for hypothalamic or pituiatry diseases Treatment of adrenal insuficiency glucocorticods: – hydrocortisone: 12-15 mg/m2 or 25 mg/day in 3 doses, or – cortisone acetate oral. 20 mg at 8 a.m. and 10 mg at 5 p.m. – prednisone p.o. 5 mg la 8 a.m. şi 2,5 mg at 5 p.m. mineralocorticoids: – 9a-fluoro-hidrocortisone (Astonin, Florinef) 0,05-0,2 mg/day Patients must avoid any stressful situation and to increase the dosage of glucocorticoids when this is anticipated, including surgery. Patients must have an identification bracelet and to have always with them a vial of hydrocortisone hemisuccinate for immediate intervention if un unexpected stressful event occurs or they have hypotension. They will consume salt following their needs. Treatment of adrenal crisis 1.Treat the precipiting factors 2. introduce an intravenous line and blood taking if the crisis occurs in a patients with unknown disease to assess: na, K, BUN, cortisole, ACTH 3. Plasma volume corection with normal saline with glucose 5 %, 3000 ml in 24 h, First 1000 ml will be given in the first 3 hours 4. Hidrocortizon hemisuccinat in a bolus of 100 mg. then 100 mg every 6 hours in the first day. The dose is rediced to 50 mg every 6 hours in the second day and then after following the patient’s evolution 5. 4-5 days after crisis the patient may be put on oral medication 6. mineralocoirticoids - 9 fluoro hidrocortizon 0,1mg-0,2 mg /zi, will be associated when gluccorticoid therapy could be reduced to 50/75 mg/day in case of unfavorable evolution 200-400 mg hydrocortisone hemisuccinate dose will be maintained until the maintaining factor is treated .