* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy
Open energy system models wikipedia , lookup
Nuclear fusion wikipedia , lookup
Energy storage wikipedia , lookup
William Flynn Martin wikipedia , lookup
100% renewable energy wikipedia , lookup
Potential energy wikipedia , lookup
Energy subsidies wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Zero-energy building wikipedia , lookup
Kinetic energy wikipedia , lookup
World energy consumption wikipedia , lookup
Regenerative brake wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Alternative energy wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy policy of Australia wikipedia , lookup
International Energy Agency wikipedia , lookup
Distributed generation wikipedia , lookup
Energy harvesting wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Internal energy wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Negawatt power wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Conservation of energy wikipedia , lookup
United States energy law wikipedia , lookup
Energy efficiency in British housing wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
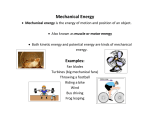
Chapter 4 – ENERGY Energy Energy—The ability to cause change and the ability to do WORK 9 Main forms of energy: – 1. Electrical: energy due to moving electrons – 2. Chemical: energy stored in bonds between atoms – 3. Thermal: energy from heat – 4. Radiant = Light: energy from the sun & light – 5. Nuclear: energy stored in the nucleus of an atom – 6. Sound: energy of vibrating sound waves – 7. Magnetic: energy of magnetism – 8. *Mechanical: Energy due to position and motion • Kinetic + Potential= Mechanical 9. Elastic potential Energy: Energy that is stored in something that stretches or compresses, such a rubber band or spring. Kinetic Energy • KE is energy of motion. • KE = 1×m×v2 2 • – m = mass (kg) – v = velocity (m/s) • SI Unit for Energy is Joule (J) –Joule = kgxm2/s2 (This is NOT an equation— just another way to write the unit) Calculating Kinetic Energy • What is the kinetic energy of a 44 kg cheetah running at 31 m/s? • KE = 1 m v2 • 2 • KE = https://www.youtube.com/watch ?v=AO117teuR2Q • Bill Nye Energy: • On the back of your paper: • Name as many examples of energy as you can from the video. • Name examples of energy changes in the video. Potential Energy (PE) • PE is energy that is stored in an object. 4 types of PE: 1. Elastic PE – Energy due to a stretch (rubber band) or compression (spring) 2. Chemical PE – Energy in the bonds between atoms (foods & fuels) 3. Gravitational PE – Energy due to an elevated position – GPE = m×g×h • m = mass (kg) • g = gravity=9.8 m/s2 (on Earth) • h = height (m) 4. Nuclear energy: Energy stored in the nucleus of an atom. Calculating Potential Energy • A 65 kg rock climber ascends a cliff. What is the climber’s GPE 35 m above the base of the cliff? • GPE= https://www.youtube.com/watch ?v=AO117teuR2Q • Bill nye energy 22 min • Bozeman science KE PE • https://www.youtub e.com/watch?v=BS Wl_Zj-CZs Law of Conservation of Energy L.o.C.o. Energy-• Energy can not be created or destroyed, it just changes form. • Rollercoaster • mass and spring Mechanical Energy • Energy can be converted from Potential to Kinetic, but the total energy doesn’t change • Total Mechanical Energy = Kinetic + Potential • ME = KE + PE Nuclear Energy • There are two types of nuclear energy • Fission and Fusion • Both forms energy are stored as mass in the atoms of certain elements. This mass can be changed into energy under the proper conditions according to Albert Einstein's famous equation: • nuclear fission • nuclear fusion • http://www.pbslearningmedia.org/search/? Fusion & Fission • Fusion atoms are "fused" together • In fusion lighter elements form heavier elements • Uses • Fission the 2nd form of nuclear energy • The exact opposite of fusion. • In fission atoms are broken apart. • In fission heavier elements form lighter elements. • Uses – Hydrogen Bomb – Nuclear Energy Plants – The middle of stars Rube Goldberg Unbelievable Rube Goldberg Machine - Google Video •