* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5
Water vapor wikipedia , lookup
Thermometer wikipedia , lookup
Atmospheric circulation wikipedia , lookup
Cold-air damming wikipedia , lookup
Tectonic–climatic interaction wikipedia , lookup
Air quality law wikipedia , lookup
Absolute zero wikipedia , lookup
Hyperthermia wikipedia , lookup
Satellite temperature measurements wikipedia , lookup
Instrumental temperature record wikipedia , lookup
Atmosphere of Earth wikipedia , lookup
Surface weather analysis wikipedia , lookup
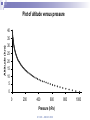
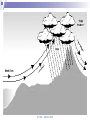
Chapter 5 Atmospheric Stability © TAFE MECAT 2008 Introduction In Chapter 4, we examined the concept of horizontal and vertical atmospheric motion, and how pressure differences create the winds that form on a variety of different scales. In this chapter, we examine the consequence of the vertical lifting of air; atmospheric stability. © TAFE – MECAT 2008 Introduction As you could predict from the terms, an atmosphere that is stable will usually bring fine weather, and one that is unstable may produce stormy weather. This chapter answers the questions of what constitutes a stable atmosphere and what changes cause a stable system to decompose to an unstable one. © TAFE – MECAT 2008 Equations of State We now need to apply all of the physical aspects of meteorology that we have learnt from the previous chapters. We will observe how buoyancy (which creates convection) and the horizontal pressure gradient force (hPGF), which creates advection) work together with pressure, temperature and density to create the stable and unstable weather we observe. © TAFE – MECAT 2008 An Equation of State Where, at sea level; = atmospheric density in kg/m3 P = atmospheric pressure in kPa R = gas constant T = atmospheric temperature in K (or virtual temperature) © TAFE – MECAT 2008 Equation of State Using this equation we can determine the unknown parameter by solving with the three known parameters. In the equation given, we are obviously solving for density, but we can easily rearrange to solve for pressure and temperature. © TAFE – MECAT 2008 Exercise You would probably have encountered equations similar to this in the laboratory calculations unit. Use the skills you have learnt to solve the following problems. Solve the above equation for density given a pressure of 99.4 kPa, and a temperature of 306 Kelvin. Rearrange the equation of state above to solve for pressure given a density of 1.18 kg/m3 and temperature of 303 Kelvin. © TAFE – MECAT 2008 Equation of State But what does all this mean? The fact that these parameters are related means that if we change one variable, we will change all the others as well We therefore need to have a thorough understanding of how they work For example, if we change the temperature, does that change the density of the air? © TAFE – MECAT 2008 The Gas Laws The gas laws describe the relationships between all of the physical variables that the atmosphere (or gases in general) exhibit. We cannot explore the behaviour of the atmosphere without understanding the basics of how gases work. Some of the relationships have been explored already, but now we need to understand the mechanisms of change by examining some of the calculations. © TAFE – MECAT 2008 Boyles Law P1V1 P2V2 Boyles law states that; If mass and temperature are kept constant, pressure and volume are inversely proportional to each other What this means is; if we increase the volume, we decrease the pressure if we increase the pressure we decrease the volume © TAFE – MECAT 2008 Charles Law V1 V2 T1 T2 Charles Law states that; Under constant pressure and mass, volume and temperature are directly proportional to each other This means that; if we increase the temperature, we increase the volume if we increase the volume we increase the temperature © TAFE – MECAT 2008 Gay-Lussac law P1 P2 T1 T2 The Gay-Lussac Law states that; Under constant mass and volume, temperature and pressure are directly proportional to each other This means that; If you increase the temperature, you increase the pressure If you increase the pressure you increase the temperature © TAFE – MECAT 2008 Avogadro’s Law V1 V2 n1 n2 Avogadro’s Law states that; Under constant pressure and temperature the volume is directly proportional to the number of moles This means that; If you increase the volume you increase the number of moles If you increase the number of moles you increase the volume This law is the part that allows us to convert between mass and moles and therefore is responsible for the density part of equations of state © TAFE – MECAT 2008 The Combined Gas Law As the name suggests, it combines various part of the other four laws. The common form is seen as; P1V1 P2V2 T1 T2 This contains Boyle’s, Charles and Gay-Lussac Laws, but derivations of the Ideal gas Law are far more appropriate for mass / mole relationships © TAFE – MECAT 2008 The Ideal Gas Law Scientist have found a better way to express the gas laws in a more universal (thermodynamic) way, called the Ideal Gas Law; PV nRT This equation can solve for almost anything, assuming you now where to put the formula weight of a compound! (with the moles of course!) © TAFE – MECAT 2008 The Ideal Gas Law This law can solve for the following; Pressure Volume Temperature Moles But, apply a formula weight, it will solve for; Molar mass Mass Density © TAFE – MECAT 2008 Exercise 5.2 This is a appropriate time to perform the gas laws task found in the Ch 5 tab of the MetExplore spreadsheet. There you will find a variety of gas laws and other interactive material that will shed light on this tricky subject! © TAFE – MECAT 2008 The Pressure / Height Relationship The second significant relationship we need to be reminded of is the pressure/height relationship. The pressure through a vertical slice of the atmosphere is not the same – it follows a curved pattern that decreases with altitude as seen in Figure 5.1 below. © TAFE – MECAT 2008 Plot of altitude versus pressure Altitude (km) 40 35 30 25 20 15 10 5 0 0 200 400 600 Pressure (hPa) © TAFE – MECAT 2008 800 1000 The Pressure / Height Relationship This is due to the compressibility of air (which creates a pressure gradient), the gravitational force, as well as the pressure gradient force. As you could imagine, physicists have created many formulas for calculating the change in pressure for any given height (MetExplore spreadsheet Ch5). © TAFE – MECAT 2008 The Effect of Water You should know by now that water has some very unique properties; a high specific heat capacity, found in all three states of matter, highest density is at 4°C, can easily sublimate under the right conditions, and there are many more. What you may not know is that water plays an enormous role in determining the stability of weather, This implies that measuring the relative humidity is important for things other than knowing how comfortable (or uncomfortable) the weather will be tomorrow! © TAFE – MECAT 2008 The Effect of Water Let’s begin by understanding how water changes its physical state, i.e. solid, liquid and gas (vapor). The change of state is controlled by temperature, obviously, solid ice melts to become liquid water at 0°C (the melting point), which boils to become a gas at 100°C (the boiling point). Figure 5.2 below shows the states of matter © TAFE – MECAT 2008 The Effect of Water What you may not know is that water not only changes state from liquid to gas at 100°C but also everywhere in-between Solid ice can change into gaseous water vapour without first becoming a liquid! This process is called sublimation © TAFE – MECAT 2008 Changes of State © TAFE – MECAT 2008 The Effect of Water But you already new this, because this non-boiling change of state is called evaporation! This vaporisation of water produces a small partial pressure which is added to all of the other partial pressures exerted by the gases in the atmosphere The pressure exerted by vaporised water in the atmosphere is called the vapour pressure of water, and is immensely important parameter in meteorology. © TAFE – MECAT 2008 The Effect of Water Water evaporates at all temperatures, but obviously more so at higher temperatures The vapour pressure of water can have many units, a common one being kPa or some other derivative. © TAFE – MECAT 2008 The Effect of Water Pressure (mm Hg) Plot of vapor pressures for liquid water 760 660 560 460 360 260 160 60 -40 274 294 314 334 Temperature (Kelvin) © TAFE – MECAT 2008 354 374 The Effect of Water One derived unit of measure that you would be very familiar with is called the relative humidity (% RH). The % RH value is a measure of the moisture content as vapour in the air. It can be calculated directly from various equations, but most people are familiar with looking the % RH value up from a humidity table (MetExplore spreadsheet under the ‘Humidity Table’ tab). Just like vapour pressure, the % RH increases with increasing temperature. © TAFE – MECAT 2008 The Effect of Water We can start to look at the reverse situation in which we see water vapour condense out of the air into liquid water. When vapor condenses to gas we call the air saturated Obviously we need a reduction in temperature for this to occur, and the temperature at which this occurs is called the dewpoint temperature. © TAFE – MECAT 2008 The Dewpoint Temperature The dewpoint temperature effectively asks the question “to what temperature will we need to reduce a parcel of air in order to achieve 100% RH (assuming a given temperature and relative humidity <100 %)”. We do this by calculating the dewpoint temperature. To understand exactly how the air temperature, dewpoint temperature and relative humidity are related, it is best to view a graph © TAFE – MECAT 2008 Dewpoint vs dry bulb Temp Relative Humidity (%) Plot of T-T d versus % RH 120 100 80 60 40 20 0 0 5 10 15 20 Difference between Air & Dewpoint Temp (T - Td) © TAFE – MECAT 2008 25 The Moisture Density Finally we need to look at how air containing moisture exhibits a change in density and how this density changes the buoyancy of an air parcel, which will eventually lead to the rising or falling of an air parcel. Figure 5.4 below shows how the density of air decreases as the vapour pressure of water increases, but why is this so! It is due to the fact that the molar mass of air is ~29, but that of water is only 18, so the more water vapor you add, the less dense the air becomes, the more buoyant air becomes. © TAFE – MECAT 2008 Plot of density versus vapor pressure 1.4 1 0.8 0.6 0.4 0.2 Vapor pressure (Pa) © TAFE – MECAT 2008 10 00 00 80 00 0 60 00 0 40 00 0 20 00 0 0 0 Density (kg/m 3) 1.2 Lifting Mechanisms in the Atmosphere Applied to the atmosphere, the term ‘stability’ simply means ‘resistant to change’. Any upwards motion causes turbulence in the air To achieve this turbulence we need to start ‘lifting’ the air, by one (or more) of four mechanisms; orographic lifting, convergence, diabatic heating and frontal systems. © TAFE – MECAT 2008 Orographic Lifting Orography is a concept in geography (specifically topography) that deals with the height of land. Mountainous terrain (can) provide a natural barrier to horizontal winds, the consequence of which is the vertical motion of air as the wind hits the mountain; it is forced to go upwards (unless is can go around). The process of a parcel or layer of air rising as a result of the topography is referred to as orographic uplifting. © TAFE – MECAT 2008 Orographic Lifting If the parcel or layer of air contains moisture then the following sequence occurs as air rise’s up the side of a mountain, the pressure and temperature decrease adiabatically until it reaches the lifting condensation level (LCL) The LCL is that point in a parcel of air when the pressure and temperature has dropped to the point were relative humidity approximates 100 %, and clouds start to form © TAFE – MECAT 2008 © TAFE – MECAT 2008 Convergence Convergence is another mechanism that can force air near the surface to rise. If winds blowing in different directions meet each other, the different moving air masses become an obstacle to one another. The air converges and has no place to go but upwards. At the surface air flows inward to the center of low pressure where it converges and then rises. Convergence also occurs when air flowing over a smooth surface suddenly hits a rougher surface and slows due to increased friction. The air piles up at the rough surface where the friction is greater, and this causes some of the air to move in a vertical direction. © TAFE – MECAT 2008 Convergence Lifting Air is forced to lift Advecting air Advecting air © TAFE – MECAT 2008 Diabatic Heating The radiation emitted by the earth (which comes from the Sun) heats the air at the surface. Air that is relatively warm compared to its surrounding rises, and it can (and usually does) cool adiabatically. As a result, the temperature drops in response to the change in pressure as per the laws of adiabatic expansion and compression. © TAFE – MECAT 2008 Diabatic Heating Heating that occurs via the sun’s radiation as diabatic heating, which is the opposite of adiabatic. The suns radiation energy is absorbed by matter (air, land, water etc), which results in the convection of warm air which expands the air, creating a parcel that is of lower density and therefore able to rise vertically through the atmosphere, and is therefore a form of lifting. You can safely assume that this is the most common form of atmospheric lifting. © TAFE – MECAT 2008 Diabatic Heating © TAFE – MECAT 2008 Frontal Systems The final lifting mechanism which we will discuss is the overriding of air at frontal boundaries. Moist air, because it is less dense, will override dry air. But how is this different from convergence? In the case of convergence, the lifting results from air molecules pushing one another upward, like pushing two small piles of sand together with your hands, forcing a larger pile to form. When two frontal boundaries meet, the lifting that occurs is due to the relative buoyancy of the two air masses. The more buoyant air mass will override the lesser buoyant air mass. The buoyancy is determined by the characteristics of the air masses (i.e. temperature and moisture content). © TAFE – MECAT 2008 Frontal Lifting Cold Front Warm Front © TAFE – MECAT 2008 Adiabatic Processes Think about where we are up to. We now know all of the significant behaviours of the atmosphere; pressure changes, temperature changes, density changes, and the relationships between these three variables; the fact that air is convecting and advecting, as well as the laws of buoyancy and adiabatic expansion and contraction. Of all of these behaviours, the most important or ‘keystone’ variable behaviour is temperature. © TAFE – MECAT 2008 Adiabatic Processes When air rise and cools (assuming adiabatically), it doesn’t just get suddenly colder, the temperature changes by a certain degree per unit of altitude, which we call a rate. Because we talk about the temperature getting cooler, we refer to the rate of change as the lapse rate, of which there are four key rates to consider; Dry Saturated Environmental, and Dewpoint © TAFE – MECAT 2008 Adiabatic Processes The key thing to remember here is that knowing the rate at which rising air cools is vital in determining the stability of the atmosphere. One important distinction should be made at this time; if a lapse rate increases, then the temperature gets colder as an air parcel rises and vice versa. To avoid confusion, we shall suggest in these notes that parcels of air rise and fall, but lapse rate increase or decrease. © TAFE – MECAT 2008 Dry Adiabatic Lapse Rate (DALR) When a parcel of air rises, it expands, and the temperature decreases. Likewise, when air sinks, it compresses, and the temperature increases. When a parcel of air, either dry or containing water vapour (<100%), rises or sinks without the addition or extraction of heat, that process is said to be a dry adiabatic process. Scientists have determined that this ‘theoretical’ lapse rate is equal to 9.8°C/km (-9.8 if the parcel rises, + 9.8 if it falls). © TAFE – MECAT 2008 DALR Plot of the Dry Adiabatic Lapse Rate 9.8 OC/km 12 Altitude (km) 10 8 6 4 2 0 -80 -60 -40 -20 Temperature (OC) © TAFE – MECAT 2008 0 20 40 Saturated Adiabatic Lapse Rate (SALR) Note that the saturated adiabatic lapse rate (SALR) is also called the moist (MALR), wet (WALR) (sometimes even called pseudo) adiabatic lapse rate. Colder air contains less water vapour due to the adiabatic and gas laws, When a parcel of air reaches its saturation point (dewpoint), condensation begins and eventually clouds will form. © TAFE – MECAT 2008 Saturated Adiabatic Lapse Rate (SALR) As water vapour condensed to liquid water at the dewpoint, latent heat is given off back to the air parcels environment, thus warming the air. In the reverse process, evaporation, we find that latent heat is taken up from the air parcel, thereby cooling the parcel of air. Since no heat is exchanged between the parcel and the environment, we still refer to this heating and cooling as an adiabatic process. © TAFE – MECAT 2008 Saturated Adiabatic Lapse Rate (SALR) However, during the processes of condensation and evaporation, the cooling and heating of the saturated parcel varies somewhat from the purely dry adiabatic process we discussed above. A rising saturated parcel cools at a slower rate due to the release of latent heat, and a sinking saturated parcel heats more slowly due to the conversion of heat energy during evaporation. This cooling of a rising (or heating of a sinking) saturated parcel is called the saturated adiabatic process which has been calculated to be an average of 6.5°C/km. © TAFE – MECAT 2008 DALR & SALR Plot of the DALR (9.8OC/km) & SALR (6.5OC/km) note that SALR is atmospheric average 12 Altitude (km) 10 8 DALR 6 SALR 4 2 0 -80 -60 -40 -20 Temperature (OC) © TAFE – MECAT 2008 0 20 40 Environmental Lapse Rate So, the DALR is a sort of theoretical, calculated ‘moistureless’ lapse rate equalling 9.8°C/km, and the SALR is a warmer lapse rate due to moisture levels approximating 6.5°C/km. So what do we get if we raise a weather balloon in the air and measure the actual temperature of the atmosphere? The easy answer is environmental lapse rate (ELR). © TAFE – MECAT 2008 Environmental Lapse Rate The problem is that the atmosphere can really be doing three things when it comes to temperature. The temperature at any point can be in a state of; Lapse Isothermal Inversion © TAFE – MECAT 2008 Environmental Lapse Rate We already know that a lapse means a decrease with altitude, so the environmental, or real, atmosphere is getting colder (indicated by a negative slope on a graph). Isothermal means ‘staying the same’, or ‘paused’, and so the temperature remains constant with altitude (indicated by a vertical line on the graph). Yet sometimes we can see an increase in temperature with an increase in altitude, and this is called a temperature inversion. These three states can be seen on the hypothetical plot in Figure 5.12 below. © TAFE – MECAT 2008 ELR Lapse Inversion Isothermal © TAFE – MECAT 2008 Dew-point Adiabatic Lapse Rate Even though a parcel of air may contain moisture, if the parcel is rising, then it cools according to the dry adiabatic lapse rate until it reaches the dew point temperature, Td. We refer to the pressure where the actual temperature equals the dew point temperature as the Lifting Condensation Level (LCL). At the LCL, the cooling process becomes a moist or saturated adiabatic process. © TAFE – MECAT 2008 Stable, Neutral and Unstable Atmospheres The stability of the atmosphere is basically determined by comparing the lapse rate of a parcel of air to the lapse rate of the surrounding air If we know the temperature and dew point of the air parcel before it begins to rise, then we can pretty accurately determine the temperature change as it rises. Measurement data provides us with a profile of the environment with which to compare our rising air. © TAFE – MECAT 2008 Stable, Neutral and Unstable Atmospheres We have found that there are three basic categories in which the atmosphere or a layer in the atmosphere can be classified in terms of stability. These categories, which we discuss next, are stable, unstable and conditional. © TAFE – MECAT 2008 © TAFE – MECAT 2008 The Stable Atmosphere As previously mentioned, a stable atmosphere is strongly resistant to changing its vertical position (regardless of the height of the atmosphere) If there was some force trying to push the air up or down, in a stable atmosphere this ‘push’ would be resisted If pushed, the parcel would move back to where it came from. © TAFE – MECAT 2008 The Stable Atmosphere Reference 2 states that “In a stable atmosphere, if you lift a parcel of air, the temperature of the rising air will decrease fast enough that its temperature will always be colder than the temperature of the environment. Colder air sinks. If the force pushing the air up suddenly disappeared, the parcel would sink back down to its original position where its temperature and pressure would be in equilibrium with the environment.” This is indicated by figure 5.13 above. © TAFE – MECAT 2008 Absolute stability occurs when ELR is less than SALR (and therefore less than DALR). This means that ELR must be lower than SALR (which varies between 3.9ºC/1km and 7.2ºC/1km) which will never be more than 7.1ºC/1km (if SALR is at its maximum). Neutral stability occurs when ELR and DALR are equal. That is, when ELR is 9.8ºC/1km. It is called 'neutral' because the thermal keeps its initial momentum and does not accelerate or slow down. This is not indicated in figure 5.13. © TAFE – MECAT 2008 The Unstable Atmosphere An unstable atmosphere is one where a parcel or layer of air is encouraged to rise or fall. In an unstable layer, the lapse rate of a rising parcel is less than the lapse rate of the environment. Because the parcel is warmer than the environment, the parcel has positive buoyancy and continues to rise on its own.2 © TAFE – MECAT 2008 The Unstable Atmosphere Absolute instability occurs when ELR is greater than DALR. We have learned that DALR is 9.8ºC/1km, therefore we can conclude that absolute instability exists when ELR is 9.9ºC/1km or greater. Meteorologists call this a "super-adiabatic lapse rate" since heat loss with elevation is so rapid.4 © TAFE – MECAT 2008 Conditional Instability To determine if the atmosphere is conditionally unstable, we compare the lapse rate of a parcel to the environment as it passes through the LCL. The atmosphere is described as conditionally unstable if the environmental lapse rate is less than the dry adiabatic lapse rate beneath the LCL and greater than the saturated adiabatic lapse above the LCL. So, conditional instability can be easily determined by making a comparison of all three lapse rates. © TAFE – MECAT 2008 Conditional Instability Conditional Instability occurs when ELR is less than DALR but more than SALR. In other words, it is when ELR is between SALR (which varies between 3.9ºC/1km to 7.2ºC/1km) and DALR (which is 9.8ºC/1km). The 'condition' for instability is only when the thermal becomes saturated, not before. © TAFE – MECAT 2008 Conclusion Atmospheric stability is not an easy concept to absorb. Overall, meteorologists use weather balloons to measure the vertical layers of the atmosphere for many parameters including temperature and pressure. From this data they produce one of four common thermodynamic diagrams to examine the atmospheres stability (SkewT-LogP, Tephigrams, Stuve Diagrams and Emagrams). © TAFE – MECAT 2008 © TAFE – MECAT 2008