* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download vaccines - Pfizer Ireland
Rotaviral gastroenteritis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Gastroenteritis wikipedia , lookup
Brucellosis wikipedia , lookup
Orthohantavirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Poliomyelitis wikipedia , lookup
Bioterrorism wikipedia , lookup
Onchocerciasis wikipedia , lookup
Cysticercosis wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Hepatitis B wikipedia , lookup
Marburg virus disease wikipedia , lookup
Leptospirosis wikipedia , lookup
Typhoid fever wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Anthrax vaccine adsorbed wikipedia , lookup
Meningococcal disease wikipedia , lookup
Whooping cough wikipedia , lookup
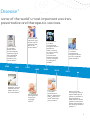
VACCINES PROTECT YOUR HEALTH AT EVERY AGE The history, facts, and future of the most effective and revolutionary public health intervention A Legacy of Achievement in Preventing Pfizer has a long history of researching and developing Our current research program includes innovative Smallpox vaccine developed at the Company’s Lancaster County Vaccine Farm.A 1882 1906 The Company manufactures one million doses of smallpox vaccine per week.B 1915 1941 The Company introduces a combined vaccine for preventing diphtheria, tetanus and pertussis in young children.B 1948 1963 First to develop bifurcated needle which subsequently revolutionizes smallpox delivery and leads to its worldwide eradication.A 1968 1968 The Company ships typhoid vaccines as part of the war effort.A The diphtheria antitoxin becomes the first FDA-licensed product manufactured at the Company’s Pearl River, New York, facility.B First to license oral form of live trivalent poliovirus vaccine in the United States.B First to develop a heat stable, freeze-dried vaccine for smallpox.A Reference Wyeth | BLederle Laboratories | CPraxis Biologics | DLederle-Praxis Biologics | EWyeth Lederle Vaccines and Pediatric | FPfizer | Pfizer. Pipeline as of November 2012. Available at http://www.pfizer.com/research/product_pipeline/product_pipeline.jsp A G 2 Disease1 some of the world’s most important vaccines. preventative and therapeutic vaccines. First to license a conjugatebased vaccine for Haemophilus influenzae type b when HibTITER is licensed for toddlers in the United States.C 1988 1991 First to license a Haemophilus influenzae type b (Hib) vaccine combined with DTP in the United States.D 1993 1999 First to license a diphtheria, tetanus, acellular pertussis (DTaP) vaccine in the United States.D First to license a meningococcal serogroup C conjugate vaccine.E First to license a 7-valent pneumoccocal conjugate vaccine [Diphtheria CRM197 Protein]), for the prevention of invasive pneumococcal disease caused by vaccine serotypes in infants and toddlers.E 2000 2010 First to license a 13-valent pneumococcal Vaccine, [Diphtheria CRM197 Protein], for use in infants and young children.F First to license a 13 valent pneumoccocal conjuguate vaccine, for use in adults 50 years and older.F 2011 Pfizer is currently investigating vaccines against Meningitis B (phase 3); Alzheimer’s disease (phase 2) Staphylococcus aureus (phase 2); Clostridium difficile colitis (phase 1) and smoking cessation (phase 1)G 3 Vaccination is the most effective and economical way of preventing infectious diseases. Vaccines have led to the control, lower incidence and even elimination of diseases in Europe that in the past resulted in death or disability for millions of people.2 – Council of the European Union, 6 June 2011 Table of Contents • What is a Vaccine? 5 – The Vaccine Revolution 5 – Life-Saving Prevention 6 – Making Vaccines: Science at Its Best 7 • Vaccines: The Key to Protecting Health at Every Age 9 – Adult Vaccination Rates – Why So Low? 9 – As We Get Older, Health Means Wealth 10 • Why Do So Many Myths Persist? 11 – The High Price of Non-Vaccination 12 – Helping Curb Bacterial Multi-Drug Resistance 12 – A Call for Better Public Defense of Vaccines 12 • The Future of Vaccines 13 • Pfizer’s Commitment to Global Health 14 • Appendix 15 – Key Dates in Vaccines History 15 – Terms and Definitions 18 • References 4 21 What is a Vaccine? A vaccine is a biological preparation which usually contains a severely attenuated, weakened or inactivate version of the virus or bacteria (also called “pathogen”) that it is meant to prevent. Introducing this biological preparation into the body prompts the immune system to combat the specific although inactive pathogen, and thus prepares the body to fight it in the future by creating an “immune memory.” As a result, a vaccinated person has the tools needed to recognize and effectively fight off a “live” version of the pathogen and prevent a potentially deadly infection.3 The Vaccine Revolution The world’s first vaccination was performed by Edward Jenner in 1796 against smallpox. A century later, Louis Pasteur developed the first rabies vaccine. Since then, scientific research and progress has led to the development of over 30 vaccines against some of the most deadly diseases known to man such as typhoid, polio, and measles.5 Thanks to effective routine childhood immunisation, diseases with serious outcomes such as polio, diphtheria or tetanus are now almost eradicated from the European Union.6 Vaccines have changed the face of medicine in just 200 years. Along with improvements in sanitation and clean water, vaccination is now recognized as the most successful & effective public health intervention in the world for saving lives and promoting good health.4 – WHO & UNICEF 5 Life-Saving Prevention Vaccines are an essential component of disease prevention. They are responsible for the global eradication of smallpox as well as saving over 3 million lives worldwide each year, and millions more from suffering illness and lifelong disability.5 Vaccines enable people to lead longer, healthier lives and also help reduce health care costs to both individuals and the broader health care system by reducing the incidence of vaccine-preventable illness.7 In addition, vaccines help protect society’s most vulnerable members – including infants, the elderly and groups at risk – from deadly diseases such as meningitis, pneumonia and measles. However there is a continuing need to develop vaccines that better address the needs of specific populations, such as those with weakened immune systems, for whom current vaccine options may not be suitable. Vaccines have eradicated some of the world’s deadliest diseases Comparison of 20th Century Annual Morbidity** and Current Morbidity: Vaccine-Preventable Diseases8 Disease Smallpox Diphtheria 20th Century Annual Rate of New Cases† 29,005 21,053 Percent Decrease 0 0 100% 100% Measles 530,217 61 >99% Mumps 162,344 2,528 98% Pertussis 200,752 21,291 89% Polio (Paralytic) 16,316 0 100% Rubella 47,745 6 >99% 152 0 100% Congenital Rubella Syndrome Tetanus Haemophilus Influenzae 580 8 99% 20,000 270* 99% Source: JAMA. 2007; 298 (18): 2155–2163. Source: CDC. MMWR January 7, 2011: 59 (52); 1704–1716. (Provisional MMWR week 52 data) † †† 2010 Reported Cases†† * 16 type b and 254 unknown serotype (<5 years of age) ** Morbidity means annual rate of new cases Comparison of Pre-vaccine Era Estimated Annual Morbidity or Mortality with Current Estimate: Vaccine-preventable Diseases8 Disease Hepatitis A Hepatitis B (Acute) Pre-vaccine Era Annual Estimate 2008 Estimate 117,333† 66,232† 11,049 11,269 91% 83% Pneumococcus (Invasive) all ages 63,067† 44,000# 30% <5 years of age 16,069† 4,167## 74% Rotavirus (Hospitalizations, <5 years of age) 62,500†† 7,500### 88% 4,085,120† 449,363 89% Varicella Source: JAMA. 2007; 298 (18): 2155–2163 †† Source: CDC. MMWR. February 6, 2009 / 58 (RR02): 1–25 # Source: CDC. Active Bacterial Core surveillance Report; S. Pneumoniae 2008. http://www.cdc.gov/abcs/survreports/spneu08.pdf † 6 Percent Decrease Source: 2008 Active Bacterial Core surveillance ### Source: New Vaccine surveillance Network ## Making Vaccines: Science at Its Best Manufacturing vaccines is a complex and highly technical process that involves stringent quality and safety procedures. Vaccines are biological products which are very sensitive to change and are highly controlled throughout the manufacturing process.9 The first step in manufacturing a vaccine is to produce the active component of the vaccine, which is usually a severely attenuated, weakened or inactivated version of the virus or bacteria, rendering it safe to administer to individuals. Manufacturing processes are then vaccine-specific. Conjugate Technology In the case of Pfizer’s 13 valent pneumococcal vaccine, thirteen polysaccharides molecules are manufactured and then bonded or conjugated with a carrier protein that helps to elicit an immune response in the immune system. The process of conjugating 13 serotypes is considerably complex as each serotype must be made separately in precise quantities to achieve the correct clinical immunological response. • Typically it takes around a year to complete the full cycle of production and testing stages for each batch of Pfizer’s 13 valent pneumococcal vaccine. • Over 300 separate quality controls tests are conducted during the production of each batch of vaccine, to help ensure that Pfizer’s 13 valent pneumococcal vaccine meets the requirements for purity, potency and safety. • Scientists perform between 10,000 to 15,000 tests per month on the manufacturing environment the vaccines are being made. Vaccines are without questions held to the single highest standard of safety than any other biological or drug. Prof. Dr. Heinz-J. SCHMITT, FIDSA, Senior Director Scientific Affairs (Vaccines) Europe, Pfizer 7 Pfizer’s Grange Castle Biotechnology Campus is one of the largest biotech manufacturing sites in the world. Pfizer recently invested $200 million in the site to introduce two new processing suites and expand current production and product testing capabilities for a number of products including Pfizer’s 13 valent pneumococcal conjugate vaccine.10 The stages in the manufacturing process Activation of the Serotypes The active components of each of the 13 vaccines contained in Pfizer’s 13 valent pneumococcal vaccine are the polysaccharides or large sugar polymers on the surface of the pathogenic bacterium, Streptococcus pneumoniae. The first step of the process involves the fermentation or growth of the organism in large tanks followed by the purification of the polysaccharide. Vaccine Conjugation The polysaccharide is then chemically bound or conjugated to a special carrier protein to form a glycoconjugate, and the glycoconjugate is then purified to a pure substance. Thirteen different glycoconjugates are manufactured for Pfizer’s 13 valent pneumococcal vaccine. Ultrafiltration and Fill After conjugation, a number of ultrafiltration steps are carried out for purity and concentration of the product before being filled. At the filling stage, the product is transferred to individual bags of conjugate. Each conjugate bag will only hold one serotype. Next, each of the 13 separate conjugates that represent the 13 serotypes found in Pfizer’s 13 valent pneumococcal vaccine is combined to create the vaccine in bulk liquid form in a process called formulation. Syringe Filling 8 After passing extensive quality control tests on a manufacturing line, Pfizer’s 13 valent pneumococcal conjugate vaccine is dispensed into syringes, in preparation for final packing labelling and distribution. Additional quality tests are conducted on the finished product. Vaccines: The Key to Protecting Your Health at Every Age Amongst adults, vaccine-preventable diseases remain a significant threat. A 2009 study reported that in the USA, significantly more adults than children die from vaccinepreventable diseases, such as influenza, pneumococcal disease, tetanus, diphtheria, pertussis (whooping cough), each year.11 In Europe, both the burden of vaccinespreventable diseases and vaccination rates amongst older adults are under-monitored. When data is available, it points to sub-optimal levels of vaccination rates. Adults were shown to be 100 times more likely to die from vaccinepreventable diseases than children in the USA.12 – Amercian College of Physicians Adult Vaccination Rates – Why So Low? 90 EU target for Influenza season 2014-15 WHO target 2010 80 Vaccination Coverage (%) 70 60 WHO target 2006 50 40 30 20 Survey 2008 Estonia Poland Lithuania Romania Slovenia Iceland Slovakia Malta Hungary Finland Portugal Norway Denmark Luxemburg Ireland Sweden Belgium Italy Spain France The Netherlands United Kingdom 0 Germany 10 Survey 2009 figure 1: Sub-optimal vaccination coverage for seasonal influenza among the elderly in the EU/EEA countries: national seasonal influenza vaccination surveys in Europe, January 2008 and July 200913 • FLU: In Europe, most countries do not reach the targeted rate of 75% vaccination rates for flu – the most well known and recognized vaccine – for older adults. • PNEUMOCCOCAL DISEASES: Immunisation rates for pneumococcal diseases amongst older adults have been low, ranging between 20% and 30%. The UK has had the highest cumulative coverage rate (over 69%), where active campaigns to promote pneumococcal vaccination for elderly adults and younger persons at risk began in 2003.14 In Europe, vaccines coverage in adults for a variety of serious vaccine-preventable diseases remains low13 and unpredictable while the burden remains high amongst older adults. 9 15 Cases/100,000 Population 12 9 6 3 0 0–4 5–14 15–24 25–44 Age Group (Years) Men 45–64 >=65 Women figure 2: The burden of pneumococcal disease amongst older adults: Notification rates for invasive pneumococcal disease by age and sex in 17 European countries in 2008 (n=12,427)15 These low and inconsistent rates of vaccination among adults have complex root causes but can be partially explained by the misplaced belief that vaccination is only for the young on the one hand, and the reliance on risk-based guidelines for adults on the other. Contrary to vaccination guidelines for young children where specific recommendations are established from birth to age five, adult vaccination recommendations tend to be patchy and focused on the existence of other diseases, such as asthma, HIV, or other diseases that affect an individual’s ability to fight off vaccinespreventable diseases. There is a growing interest and discussion around establishing European best-practice guidelines which would mimic the model and hence the effectiveness of childhood immunisation schedules. Such “age-based” guidelines would be more easily understood by both practitioners and patients, and would be easier to implement than a multitude of risk-based guidelines which require identifying individuals with specific diseases and usually fail to achieve high coverage levels.14 As We Get Older, Health Means Wealth Europe’s population is ageing fast and has the highest proportion of “older people (60+)” of all continents.16 Currently four people of working age support one “retiree”. By 2050, this ratio will drop to two for every retired person.17 The European Commission estimates that member states health care spending will increase by approximately 25% (gross domestic product) in most Member States due to the growing number of older citizens alone.18 As governments push back retirement ages to meet this future challenge, prevention strategies such as adult vaccination programs have the potential to keep Europe’s senior population active and healthy. 100% 1.2 2.0 7.9 10.7 80% 15.2 60% 3.4 6.5 11.8 12.3 15.4 16.2 18.5 17.2 21.3 35.0 18.5 32.7 36.9 40% 31.1 15.8 13.0 24.9 23.7 17.1 0% 1950 1975 80+ 65–79 2000 50–64 10.5 9.7 14.4 13.3 2025 25–49 15–24 figure 3: Population distribution in EU25 by age group (1950-2050).19 10 28.2 15.5 20% 2050 0–14 – UN Why Do So Many Myths Persist? Despite the success of childhood immunisation programs, and undisputable evidence that vaccines are safe and effective, false and misleading information continues to circulate regarding vaccines in general. This misinformation leads people to question the value of vaccines, resulting in a drop myths in vaccination rates and new outbreaks of old diseases. Both the European Centre for Disease Prevention & Control (ECDC) and the World Health Organization (WHO) have called on all stakeholders to partner together to counter common myths and inaccuracies.20 facts •“People who get vaccinated for the flu often wind up getting it.” •This is false. Certain vaccines can lead to mild symptoms resembling the disease against which they provide protection, but those are mainly a consequence of the immune system gearing up to produce the immune response.21 •“Vaccines cause side effects, illnesses, and even death – not to mention possible long-term effects we don’t even know about.” •The vast majority of side effects are minor and temporary, such as a sore arm or mild fever, and have nothing to do with the infectious disease against which the immunisation is directed.22 New vaccines go through a rigorous testing in development and approval phases in Europe, to make sure they are safe, effective and of good quality.23 The European Medicines Agency also monitors any adverse events that might occur after licensure of the medicine. •“Vaccine-preventable diseases have been •This is one of the most damaging myths today. Past virtually eliminated from Europe, so vaccination programs do not eliminate the need for adults, there is no need for my child or myself to young people and children to be vaccinated today. The be vaccinated.” indirect protection against disease (also called “herd immunity”), only results when a sufficient number (from 85% to 95% depending on the disease24) of individuals in a community are immuned to that disease. For example, diphtheria requires 85 percent of the population to be vaccinated, while measles and mumps immunization levels must approximate 92–96% and 88–92% respectively before herd immunity becomes effective.25 Routine childhood vaccines effectively protect between 85% and 95% of recipients against the targeted diseases.24 – Fine & Mulholland 1111 The High Price of Non-Vaccination Unfortunately, all of these misperceptions have led some individuals to avoid vaccinations for either themselves or their family members, and the cost to individuals and society is measurable and high. Bacterial multi-drug resistance causes 25,000 deaths per year in the EU.31 – ECDC • New outbreaks of old diseases While most vaccines-preventable diseases’ prevalence is being kept at very low levels, recent outbreaks of measles, and even poliomyelitis, across Europe attest to the fact that these diseases still pose a threat if vaccination coverage is not kept high.26 • High cost of hospital stays and lost days of work The annual cost-burden for treating pneumonia alone across Europe is 10 billion Euros mostly due to in-hospital care and lost work days.27 Helping curb bacterial multi-drug resistance While some vaccine-preventable diseases can be treated with antibiotics, over-utilisation of antibiotics leads to drug resistance, which increases the risk of infectious disease outbreaks and the potential for widespread illness.29 Since the mid-1990s the proportion of pneumococci bacteria resistant to antibiotics has increased in many European countries from a few percent to 5– 35%.30 These cases are estimated to result in extra healthcare costs and productivity losses of at least EUR 1.5 billion each year, a burden likely to increase as numbers rise.31 Encouraging vaccination along with more appropriate use of antibiotics can lower drug resistance rates by reducing the ability of infectious agents to develop resistance mechanisms. A Call For Better Public Defense Of Vaccines Healthcare professionals and public health officials are equally concerned with the rise of antibioticresistant bacteria and the dwindling number of antibiotics that remain to treat infections. Vaccines information should be part of the development of a European public health communication strategy that allows European citizens to better understand the value of vaccines, assess their individual risk in relation to vaccines-preventable illness and manage their own health. 12 12 In 2008, 90% of the people who contracted measles in EU/EEA countries had not been immunised.28 – ECDC – ECDC The Future of Vaccines Future vaccines will not only prevent infectious diseases, but research is also on-going into “therapeutic” vaccines to cure such chronic diseases as diabetes, Alzheimer’s and nicotine dependence. It’s expected that vaccines will go beyond their traditional prevention remit to treat autoimmune diseases and allergic disorders. In addition, work is also underway to develop new ways of administering vaccines like transcutaneousA and orally. Those medical and technological advances are void if not coupled with comprehensive programs to ensure full understanding of the benefits and safety of vaccines, as well as lifecourse vaccination guidelines to ensure wide and timely access to these vaccines. The first decade of this century has been the most productive in the history of vaccine development.32 – WHO 200 candidates in development33 Pandemic influenza Cervical cancer, vulval and vaginal cancer, Genital warts, Shingles, Rotavirus gastroenteritis Pneumococcal disease (conjugate vaccine) Meningococcal C disease (conjugate vaccine) Hepatitis A Chickenpox, Whooping cough (acellular vaccine) Haemophilus influenzae type b Japanese encephalitis Hepatitis B Pneumococcal disease (polysaccharide vaccine) Rubella Anthrax Mumps Measles Polio (Oral vaccine) Polio (Injectable vaccine) Tickborne encephalitis, Rickettsia typhus Clostridim difficile Cytomegalovirus virus Dengue fever Ebola virus Enterotoxigenic Escherichia coli Herpes simplex HIV Malaria Meningococcal B disease SARS Staphylococcus aureus Tuberculosis Addiction (Cocaine, Nicotine) Yellow fever, Influenza Whooping cough, Tuberculosis, Tetanus Allergies Alzheimer’s Cancer Multiple sclerosis Parkinsons Diphtheria Plague Typhoid, Cholera Rabies Smallpox 1800 A Tomorrow 1840 1880 1920 1930 1940 1950 1960 1970 1980 1990 2000 2010 Delivery of vaccine to the skin, via patches for instance. 13 Pfizer’s Commitment to Global Health Pneumococcal diseases overwhelmingly burdens young children and the elderly34, and kill over 1.6 million people each year – including approximately 800,000 children before their 5th birthday35. More than 90% of these deaths occur in developing countries.36 Through the Advanced Market Commitment (AMC) program, Pfizer reaffirmed its commitment to working with the global health community to accelerate global access to its vaccines in the world’s poorest countries on an affordable and sustainable basis. As of December 2011, Pfizer’s 13 valent pneumococcal conjugate vaccines is available in more than 85 percent of countries which have launched pneumococcal immunisation programs under the AMC with many additional launches planned.37 To meet the growing global demand, Pfizer is investing resources to increase its manufacturing capabilities and in the development of a preserved, multidose vial which, subject to WHO prequalification, is expected to offer an alternative option for developing countries.38 For more information on the GAVI Alliance, visit: http://www.gavialliance.org/ For more information on Pfizer’s vaccines activities, visit: www.pfizer.com For more information on vaccines in Europe, visit: www.ecdc.europa.eu http://www.euro.who.int/en/home http://www.historyofvaccines.org/ Pfizer has pledged to supply up to 480 million doses of its 13-valent pneumococcal conjugate vaccine through 2023 in developing countries, under the auspices of the Advance Market Commitment (AMC), an innovative program piloted by the GAVI Alliance to enable affordable access to medicines by developing countries.39 14 Appendix Key Dates in Vaccines History Scientific History of vaccines Introduction of Vaccines: historical time-line (1796-2004)42 Thucydides notices that smallpox survivors do not get reinfected.40 429 BC * Chinese discover variolation–a primitive form of vaccination aimed at preventing smallpox by exposing healthy people to tissue from the scabs caused by the disease.40 900 AD Smallpox (liveattenuated) Dr Edward Jenner, British physician, discovers vaccination40. The first smallpox vaccination is created. Vaccination became popular throughout Europe and, soon after, the US41. Rabies (killedinactivated)* Cholera (killedinactivated)* Typhoid (killedinactivated)* Diphtheria (D) (toxoid) Pertussis (Pw), whole cell (killedinactivated) Tetanus (T) (toxoid) Tuberculosis (BCG) (liveattenuated). 1796 1885 Yellow fever (liveattenuated) Influenza (killedinactivated)* Tickborne encephalitis (killedinactivated)* 1928 1936 1937 Japanese B encephalitis (killedinactivated) Polio (IPV) (killedinactivated) DTPw Polio (OPV), oral polio vaccine (liveattenuated) DT-IPV Measles (M) (liveattenuated) DTPw-IPV 1945 1955 1958 1961 1963 1966 Mumps (M) (live-attenuated) Rubella (R) (liveattenuated) MMR Meningococcal disease (serogroup A – polysaccharide) Meningococcal disease (serogroup C – polysaccharide) Meningococcal disease (serogroups A&C – polysaccharide) Pneumococcal disease (14 serotypes – polysaccharide) 1967 Replaced by a new vaccine 15 Typhoid (liveattenuated)* Hepatitis B (HB) (protein) Pertussis acellular (Pa) 1981 Pneumococcal disease (23 serotypes – polysaccharide) Varicella-zoster (live-attenuated) HB (protein/ recombinant DNA) Influenza (subunit) Haemophilus influenzae type b (Hib) (polysaccharideprotein conjugate) Typhoid (polysaccharide) Hepatitis A (HA) (killed-inactivated) DTPw-IPV-Hib DTPa HA (subunit, adjuvanted) DTPw-HB HB-HA DTPa-Hib DTPa-IPV-Hib 1983 Rotavirus (live attenuated) HPV vaccine approved in Europe. 2004 16 2006 Influenza (subunit, adjuvanted) DTPa Meningococcal disease (serogroup C– polysaccharide-protein conjugate) HA-Typhoid (polysaccharide) dTaP-IPV DTPa-IPV-HB DTPa-IPV-HBHib Pneumococcal disease (7 serotypes – polysaccharide-protein conjugate) Pseudomonal disease (8 serotypes – polysaccharide-protein conjugate) Influenza (live-attenuated) Meningococcal disease (serogroups ACW – polysaccharide) 1997 1999 2000 2001 2003 Pandemic Influenza vaccine approved in Europe. pneumoccocal conjugate vaccine, 13-valent, licensed in Europe for infants and young children. 2009 2009 pneumoccocal conjugate vaccine, 13-valent, licensed in Europe for adults 50+. 2011 Epidemiological, social & political history of Vaccines 1700s Variolation spreads around the world. At this time, smallpox was the most infectious disease in Europe, killing up to one-fifth of those infected in numerous epidemics. Variolation caused mild illness but, although it occasionally caused death, smallpox rates were lower in populations that tried it.40 Sep. 20102011 measles outbreak in Europe.45 1870s Violent opposition to vaccination. People found it hard to believe that it really worked. They also felt that it took away people’s civil liberties, particularly now that it was compulsory.40 1956 WHO launches global drive to eradicate smallpox.40 Sep. 2010 2010 Member States in the WHO European Region set themselves 2015 as new target for eliminating measles and rubella.46 Reemerging cases of Polio in Europe.47 1974 Launch of the World Health Organization’s Expanded Program on Immunisation (EPI), including six vaccines, covering diphtheria, tetanus, whooping cough, measles, polio, and tuberculosis. Before 1974, only 5 per cent of children were vaccinated against these diseases. Today over 70 per cent are vaccinated.43 1980 Smallpox eradicated from the world.40 1988 WHO launches global drive to eradicate polio by the year 2000.41 1999 2000 WHO set up a Global Advisory Committee on Vaccine Safety, made up of independent experts, to respond promptly, efficiently, and with scientific rigour, to rumours and reports related to vaccine safety.44 Global Alliance for Vaccines and Immunisation established to strengthen routine vaccinations and introduce new and underused vaccines in countries with a per capita GDP of under US$1000. GAVI is now entering its second phase of funding, which extends through 2014 17 Terms and Definitions Adjuvants: A substance (e.g. aluminum salt) that is added during production of a vaccine to increase the body’s immune response to a vaccine.48 Antibody: A protein found in the blood that is produced in response to a foreign substance (e.g., bacteria or viruses) invading the body. Antibodies protect the body from disease by binding to these organisms and destroying them.48 Antigens: Foreign substances (e.g., bacteria or viruses) in the body that are capable of causing disease. The presence of antigens in the body triggers an immune response, usually the production of antibodies.48 Attenuated Vaccine: A vaccine created by reducing the virulence of a pathogen but still keeping it ‘live’ so as to ensure an immune response. The aim is to render the living agent harmless or less virulent.49 Conjugate vaccine: A vaccine in which two compounds (usually a protein and polysaccharide) are joined together to increase a vaccine’s effectiveness. Conjugate vaccines include certain pneumococcal and meningococcal vaccines.50 Herd immunity: Indirect protection against disease that results from a sufficient number (from 85 to 95% depending on the disease24) of individuals in a community having immunity to that disease. Herd immunity does not apply to diseases such as tetanus, that are not spread via person-to-person contact.51 Immunogen: A substance able to provoke an immune response. “Immunogenicity” refers to an immunogen’s ability to provoke such a response under particular circumstances.51 Immunotherapeutic vaccines: vaccines designed to treat against conditions beyond the infectious diseases or cancer arena. Most of the candidates are still in early-stage development and their underlying scientific bases require additional investigation: Smoking cessation52, Alzheimer’s disease; 18 Allergies; Diabetes; Atherosclerosis Hypertension53; Multiple Sclerosis . Inactivated vaccine: A type of vaccine in which the vaccine pathogen (a viral or bacterial) is killed or otherwise altered so that it cannot cause infection, but can still provoke an immune response.51 Pathogen: A microorganism capable of producing a disease (e.g., bacterium or virus).54 Prophylactic vaccine: A biological preparation intended to prevent individuals from contracting given diseases. Therapeutic vaccines: vaccines which intend to treat chronic infections by inducing immune responses that suppress infection, even when the host has been unable to create those responses naturally.52 Selected vaccines-preventable diseases: Diphtheria Diphtheria is an acute disease caused by toxin-producing strains of Corynebacterium diphtheriae bacteria, that is known to colonise mucous membranes.55 Diphtheria affects people of all ages, but most often it strikes unimmunized children. In 2,000, 30,000 cases and 3,000 deaths of diphtheria were reported worldwide.56 Haemophilus influenzae type b infection Haemophilus influenzae serotype b (Hib) is the most common cause of bacterial meningitis in children aged two months to five years, in those countries where suitable vaccination programmes are not in place.55 Hib is estimated to cause millions of cases of serious disease worldwide and hundreds of thousands of deaths each year among young children.57 Hepatitis A: Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). Hepatitis A can affect anyone.58 Hepatitis B: is a serious disease caused by a virus that attacks the liver. The virus, which is called hepatitis B virus (HBV), can cause lifelong infection, cirrhosis (scarring) of the liver, liver cancer, liver failure, and death.59 human papillomavirus (HPV), HPV is a common infection: over three quarters of sexually active women are estimated to be infected at least once in their lifetimes. Persistent infection with oncogenic HPV types can cause cervical cancer in women and anogenital cancers in both sexes.60 Influenza (seasonal): Influenza (the flu) is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness, and at times can lead to death. Some people, such as older people, young children, and people with certain health conditions, are at high risk for serious flu complications.61 Measles: Measles is highly infectious vaccinespreventable disease caused by morbillivirus. The disease is transmitted via airborne respiratory droplets, or by direct contact with nasal and throat secretions of infected individuals.55 Measles is one of the leading causes of childhood mortality, leading to about 240,000 deaths worldwide each year.62 Meningococcal infection: Meningococcal infection is caused by Neisseria meningitidis, a bacterium with human carriers as the only reservoir.55 Meningococcal group C (or Meningitis C) is one of the 6 groups of the bacteria Neisseria meningitides which can cause meningitis and septicaemia. Mumps: Mumps is an acute illness caused by the mumps virus. It is characterised by fever and swelling of one or more salivary glands.55 Mumps is mostly a mild childhood disease. It most often affects children between five and nine years old.63 Pertussis: Pertussis is an acute bacterial infection of the respiratory tract caused by the bacterium Bordetella pertussis. The disease is characterised by a severe cough.55 Pertussis (whooping cough) is an important cause of infant death worldwide and continues to be a public health concern even in countries with high vaccination coverage. Estimates from WHO suggest that, in 2008, about 16 million cases of pertussis occurred worldwide, 95% of which were in developing countries, and that about 195,000 children died from the disease.64 Pneumococcal infection: [Pneumococcal Disease (PD) describes a group of illnesses caused by the bacterium Streptococcus pneumoniae (S. pneumoniae), also called pneumococcus, and can include invasive infections such as meningitis, bacteremic pneumonia as well as noninvasive infections such as non-bacteraemic pneumonia and acute otitis media.65 According to a WHO 2004 estimate, PD is the leading cause of vaccinepreventable death in children younger than 5 years of age worldwide.66 The proliferation of resistant strains of S pneumoniae and other pathogens in the past 15 years threatens the successful treatment of CAP67 Pneumoccocus vaccine is deemed underused by the WHOEurope Office.68 Poliomyelitis: Polio is caused by polioviruses, classified into types 1, 2 and 3. Humans are the only reservoir of infection. Transmission occurs via the oral-faecal route or contact with saliva.55 Polio is a highly infectious and sometimes fatal disease, which invades the nervous system and can cause paralysis in a matter of hours. The disease usually affects children under 5 years of age. In June 2002, all 53 countries in the WHO European Region were certified polio free. Since then, the region has experiences several episodes of wild poliovirus importation but sustained effort of immunisation and disease surveillance helps maintain the Region’s polio-free status.69 Rubella: Rubella is a mild febrile rash illness caused by rubella virus. It is transmitted from person to person via droplets (the virus is present in throat secretions). It affects mainly, but not only, children and can often lead to serious and sometimes fatal complications in the fetus when an unprotected woman acquires the infection early in pregnancy. Humans are the only reservoir of infection.55 Rotavirus: Rotavirus infection affects nearly all the children of the world. It causes about 25% of all diarrhoeal illnesses in children under 5, and is a major cause of morbidity and mortality globally. According to WHO estimates, more than 10,000 children under 19 5 die each year in the WHO European Region due to rotavirus infection. WHO/Europe works with Member States to accelerate the introduction of rotavirus vaccine into their national immunisation programmes, and to establish a regional surveillance network to collect local data on disease burden and monitor the impact of vaccines.70 Shingles (Herpes Zoster): Shingles is a painful localized skin rash often with blisters that is caused by the varicella zoster virus (VZV), the same virus that causes chickenpox. Shingles most commonly occurs in people 50 years old or older, people who have medical conditions that keep the immune system from working properly, or people who receive immunosuppressive drugs.71 Smallpox: Smallpox is a serious, contagious, and sometimes fatal infectious disease caused by the variola virus and characterized by extensive rash and higher fever.72 The global eradication of smallpox was certified in 1980.73 Tetanus: Tetanus is an often fatal disease, which is present worldwide, and affects people of all ages but is particularly common and serious in newborn babies. It is a consequence of a toxin produced by the bacterium Clostridium tetani. The main reservoirs of the bacterium are herbivores, which harbour the bacteria in their bowels (with no consequences for them) and disseminate the “spore form” of the bacteria in the environment with their faeces.55 WHO estimated that neonatal tetanus killed about 180,000 babies in 2002.74 Varicella infection (chickenpox): Chickenpox is caused by the varicella-zoster virus (VZV), which also causes shingles. The virus spreads through the body into the skin causing rashes to appear.55 Yellow Fever: Yellow fever is caused by the yellow fever virus, which is carried by mosquitoes. It is endemic in 33 countries in Africa and 11 countries in South America.75 Other diseases against which Pfizer is investigating vaccines: Alzheimer’s disease: a degenerative disease, which slowly and progressively destroys brain cells, affecting memory and mental 20 functioning.76 In 2010, 9.95 million individuals were suffering from Alzheimer in Europe and 35.6 million people worldwide.77 Predictions show the number will double over the next 20 years.78 Alzheimer also has an economic cost for society, which has been estimated at US$604 billion worldwide in 2010, comprising the cost of informal care, direct cost of social care, and the direct cost of medical care.79 This huge burden of disease is yet unaddressed as neither a preventative nor a curative treatment exist to date. Pfizer is currently investigating a vaccine in phase 2.80 Staphylococcus aureus: ‘Staphylococcus aureus is an opportunistic bacterial pathogen associated with asymptomatic colonization of the skin and mucosal surfaces of normal humans. It is the most frequently isolated bacterial pathogen from patients with hospitalacquired infections. Before the introduction of antimicrobials in the 1940s, the mortality rate of S. aureus invasive infection was about 90%. The initial success of antibiotherapy was rapidly countered by the successive emergence of penicillin-resistant, then methicillin-resistant S. aureus (MRSA) strains, and since 2002 by that of vancomycin-resistant strains.81 ’Pfizer is currently investigating a vaccine in phase 2. Meningitis B: Meningitis is an infection of the meninges, the thin lining that surrounds the brain and spinal cord, caused by the bacteria Neisseria meningitidis group B, one of 6 groups of bacteria which cause disease.82 ‘Serogroup B accounts for up to 80% of meningococcal disease in European countries such as UK, Norway, The Netherlands, Germany and Denmark.’83 Pfizer is currently investigating a vaccine in phase 3.80 Clostridium difficile colitis: is a bacterium that causes inflammation of the colon, known as colitis. People who have other illnesses or conditions requiring prolonged use of antibiotics, and the elderly, are at greater risk of acquiring this disease. The bacteria are shed through feces.84 Pfizer is currently investigating a vaccine in phase 1.80 Smoking Cessation: Pfizer is currently investigating a vaccine for smoking cessation in phase 1.80 References 1. Pfizer Inc. Vaccines: Legacy of Achievement webpage. Available at http://www.pfizer.com/health/vaccines/index.jsp 2. Council of the European Union, press release 3095th Council meeting, Employment, Social Policy, Health and Consumer Affairs—Health issues, Luxembourg, June 6, 2011, p.9. 3. Adapted from WHO Vaccines webpage and http://www.vaccinestoday.eu/glossary/vaccine/ 4. WHO, UNICEF. Global Immunization Vision & Strategy 2006-2015. Geneva/New York, 2005, p.3. 5. WHO, UNICEF, World Bank. State of the World’s Vaccines and Immunization, 3rd Edition. Geneva. November 2009. Available at http:// whqlibdoc.who.int/publications/2009/9789241563864_eng.pdf. 6. ECDC. Annual Epidemiological Report 2010, Stockholm: ECDC; 2010, p. 5. 7. Pfizer Inc. Vaccines-value, Policy Purple Paper, October 2011. 8. National Centre for Immunisation & Respiratory Diseases; CDC, 2010. 9. EVM. Animal welfare and vaccine development, position paper, September 2008. 10. Pfizer. Pfizer invests $200 million in its Grange Castle biotech site, press release, September 22, 2011. Available at http://www.pfizer.ie/in_the_ news.cfm?action=showArticle&articleID=83 11. Fingar AR, Francis BJ. “American College of Preventive Medicine Practice Policy Statement: Adult immunizations.” Am J Prev Med. 1998;14:156158; Poland GA, Jacobson RM, Ovsyannikova IG. “Trends affecting the future of vaccine development and delivery: The role of demographics, regulatory science, the anti-vaccine movement, and vaccinomics.” Vaccine. 2009, doi:10.1016/j.vaccine.2009.01.069. 12. American College of Physicians. Adult Immunisation webpage, last accessed on June 27, 2012. 13. Mereckiene J. et al. on behalf of the VENICE project gatekeepers group. “Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008-2009.” Euro Surveill. 2010;15(44). 14. David S Fedson et al. “Pneumococcal polysaccharide vaccination for adults: New perspectives for Europe.” Expert Rev Vaccines. 2011;10(8):11431167. 15. ECDC. Surveillance Report. Annual Epidemiological Report on Communicable Diseases in Europe 2010. Available at www.ecdc.europa.eu/en/ publications/Publications/1011_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf. 16. United Nations. World Population Ageing 2009. New York: United Nations, Department of Economic and Social Affairs, Population Division, 2010, p.13. 17. United Nations. World Population Ageing 2009. New York: United Nations, Department of Economic and Social Affairs, Population Division, 2010, p.23. 18. DG ECFIN. The Impact of Ageing on Public Expenditure: Projections for the EU25 member States on Pensions, Health Care, Long Term Care, Education and Unemployment Transfers (2004-2050), 2006, p.133. 19. Commission of the European Communities , Green Paper “Confronting Demographic change: a new solidarity between Generations”, Brussels 16.3.2005, p.19. 20. WHO, UNICEF, World Bank. State of the World’s Vaccines and Immunization, 3rd Edition. Geneva, WHO, 2009, p.66. ECDC. Annual Epidemiological Report 2010, Stockholm: ECDC; 2010, p.5. 21. http://www.vaccinestoday.eu/facts/11-do-vaccinations-cause-the-diseases-they-are-designed-to-prevent/ 22. CDC. Some Common Misconceptions and How to Respond to Them. Available at http://www.cdc.gov/vaccines/vac-gen/6mishome.htm, last accessed on December 21, 2011. 23. WHO. Vaccine Safety and Quality webpage. Available at http://www.who.int/immunization_safety/safety_quality/en/. 24. Paul E.M. Fine, Kim Mulholland. “Community immunity.” Vaccines. S. Plotkin, W. Orenstein, and P. Offit, eds. 5th Edition. Philadelphia, PA: Elsevier, 2008. 25. Paul E.M. Fine, Kim Mulholland. “Community immunity.” Vaccines. S. Plotkin, W. Orenstein, and P. Offit, eds. 5th Edition. Philadelphia, PA: Elsevier, 2008. Anderson Rm, May Rm. “Vaccination and herd immunity to infectious disease.” Nature. 1985; 318: 323-329. 26. ECDC. Annual Epidemiological Report 2011, Reporting on 2009 Surveillance Data and 2010 Epidemic Intelligence Data. Stockholm: ECDC; 2011, p. 6 & 163. 27. Welte T. et al. “Clinical and economic burden of community-acquired pneumonia in adults.” Thorax. 2012;67:71-79. 28. ECDC. Measles Elimination within Reach webpage, http://ecdc.europa.eu/en/healthtopics/spotlight/Spotlight_immunisation/Pages/Vaccines_ powerful_tools.aspx, accessed on April 2011. 29. WHO. Anti-microbial Resistance Factsheet, No. 194, February 2011. 30. Garcia-Rey C, Aguilar L, Baquero F, Casal J, Dal-Re R. “Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae.” J Clin Microbiol. 2002;40(1):159-164. Perez-Trallero E. et al. “Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: Results of a 1-year (1998-1999) multicenter surveillance study in Spain.” Antimicrob Agents Chemother. 2001 Dec;45(12):3334-3340. Reinert RR. et al. “Emergence of macrolide and penicillin resistance among invasive pneumococcal isolates in Germany.” J Antimicrob Chemother. 2002 Jan;49(1):61-68. 31. ECDC/EMEA. Joint Technical Report: The bacterial Challenge: Time to React., Stockholm, September 2009, p. vi. 32. WHO, UNICEF, World Bank. State of the World’s Vaccines and Immunization, 3rd Edition. Geneva, WHO, 2009, p.XXI. 33. Adapted from European Federation of Pharmaceutical Industries and Association, Medicines for Mankind-Today’s research, Tomorrow’s CureBetter Health Through Vaccination, 2004. (2) EVM brochure, April 2008 ; (3) IFPMA brochure 2008. 34. WHO. Pneumoccocal Disease webpage. Available at http://www.who.int/immunization/topics/pneumococcal_disease/en/index.html 21 35. WHO/2007/ 94/C/7-10; WHO/ 2004/Para D. 36. Bogaert D, de Groot R, Hermans PWM. “Streptococcus pneumonia colonization: The key to pneumococcal disease.” The Lancet Infect Dis. 2004; 4: 144-154. 37. Pfizer Inc. Pfizer in the developing world, Purple Policy Paper, December 2011. 38. Pfizer Inc. Advance Market Commitment for pneumococcal Conjuguate Vaccine: Helping to prevent pneumococcal deaths among children in the poorest nations. Leaflet no 401006, September 2011. 39. Pfizer Inc. Press release, Pfizer Broadens and Extends Commitment to Help Prevent Pneumococcal Disease in Infants and Young Children in the World’s Poorest Countries, December 16, 2011. Available at http://embu.pfizer.com/AFME/Pages/ PfizerBroadensAndExtendsCommitmentToHelpPreventPneumococcalDiseaseIn.aspx. Pfizer Inc. Pfizer in the developing world, Purple Policy Paper, December 2011. 40. NHS. The History of Vaccination. Available at http://www.nhs.uk/Planners/vaccinations/Pages/historyofvaccination.aspx 41. CDC. Vaccines Timeline, 50 Years of Vaccine Progress, April 12, 2005. 42. EFPIA. Medicines for Mankind-Better Health through Vaccination, September 2004. 43. David E. Bloom, David Canning, Mark Weston. “The value of vaccination.” World Economics. 2005; 6(3):19. 44. WHO, UNICEF, World Bank. State of the World’s Vaccines and Immunization, Third Edition. November 2009, p.67. Available at http://whqlibdoc. who.int/publications/2009/9789241563864_eng.pdf. 45. WHO. Measles Outbreak in Europe, April 21, 2011. Available at http://www.who.int/csr/don/2011_04_21/en/ 46. WHO-Europe. Next Steps towards Eliminating Measles and Rubella in Europe, December 17, 2011. Available at http://www.euro.who.int/en/ what-we-do/health-topics/communicable-diseases/measles-and-rubella/news/news/2010/16/next-steps-towards-eliminating-measles-andrubella-in-europe 47. WHO-Europe. Immunization at Crossroads in Europe, March 3, 2011. Available at http://www.euro.who.int/en/who-we-are/regional-director/ news/news/2011/03/immunization-in-europe-at-crossroads. 48. CDC. Vaccines & Immunizations: Glossary. Available at http://www.cdc.gov/vaccines/about/terms/glossary.htm. 49. VaccinesToday. Attenuated Vaccine. Available at http://www.vaccinestoday.eu/glossary/attenuated-vaccine/ 50. VaccinesToday. Conjugate Vaccine. Available at http://www.vaccinestoday.eu/glossary/CDC. Vaccines & Immunizations: Glossary. http://www. cdc.gov/vaccines/about/terms/glossary.htm. 51. The History of Vaccines website, Glossary of Terms. Available at http://www.historyofvaccines.org/glossary 52. Stanley A Plotkin. “Vaccines: Past, present, future.” Nature Medicine. 2005; 11(4). 53. Datamonitor. Infectious Diseases Vaccine Market Overview: Key Companies & Strategies, October 2010, p.26. 54. Venes D, ed. Taber’s Cyclopedic Medical Dictionary, 19th Edition. Philadelphia, PA: F.A. Davis Company, 2001. 55. ECDC. Immunisation. Available at http://ecdc.europa.eu/en/healthtopics/immunisation/Pages/index.aspx 56. WHO. Diphteria. Available at http://www.who.int/immunization/topics/diphtheria/en/index.html 57. WHO-Europe. Haemophilus Influenza (HiB) Disease. Available at http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/ haemophilus-influenza-hib-diseases 58. CDC. Hepatitis A Vaccination. Available at http://www.cdc.gov/vaccines/vpd-vac/hepa/default.htm 59. CDC. Hepatitis B Vaccination. Available at http://www.cdc.gov/vaccines/vpd-vac/hepa/default.htm 60. WHO-Europe. Preparing for the Introduction of HPV Vaccine in the WHO European Region. Available at http://www.euro.who.int/en/what-we-do/ health-topics/disease-prevention/vaccines-and-immunization/publications/pre-2009/preparing-for-the-introduction-of-hpv-vaccine-in-the-whoeuropean-region 61. CDC. Seasonal Influenza: Flu Basics. Available at http://www.cdc.gov/flu/about/disease/ 62. WHO-Europe. Measles & Rubella. Available at http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/measles-andrubella/news2/news/2010/16/next-steps-towards-eliminating-measles-and-rubella-in-europe/measles-and-rubella-serious-diseases 63. WHO. Mumps. Available at http://www.who.int/immunization/topics/mumps/en/index.html 64. WHO. Pertussis. Available at http://www.who.int/immunization/topics/pertussis/en/index.html 65. CDC Pink Book 2008/ 218/F and 219/M ; CDC/ 1997/1/”Introduction”/1-7. 66. MMWR 2006/pg 512/B/15-17 WHO/ 2007/ 94/ C/7-10 WHO/2004/ Para D. 67. Bruinsma N. et al. “Trends of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae in Europe.” J Antimicrob Chemother. 2004;54:1045-1050. 68. WHO-Europe. Underused vaccines. Available at http://www.euro.who.int/en/what-we-do/health-topics/disease-prevention/vaccines-andimmunization/vaccine-preventable-diseases/underused-vaccines 69. WHO-Europe. Poliomyelitis. Available at http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/poliomyelitis 70. WHO-Europe. Rotavirus. Available at http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/rotavirus 71. CDC. Shingles Vaccination. Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/default.htm 72. CDC. Smallpox Disease Overview. Available at http://www.emergency.cdc.gov/agent/smallpox/overview/disease-facts.asp. 73. WHO. Smallpox Factsheet. Available at http://www.who.int/mediacentre/factsheets/smallpox/en/ 22 74. WHO. Tetanus. Available at http://www.who.int/immunization/topics/tetanus/en/index.html 75. WHO. Yellow Fever. Available at http://www.who.int/immunization_monitoring/diseases/yellow_fever/en/index.html 76. Alzheimer Europe. Alzheimer’s Disease webpage. Available at http://www.alzheimer-europe.org/index.php/EN/Dementia/Alzheimer-s-disease 77. Alzheimer’s Disease International. World Alzheimer Report 2010, p.15. Available at http://www.alz.org/documents/national/World_Alzheimer_ Report_2010.pdf. 78. Ferri et al. “Global prevalence of dementia: A Delphi consensus study.” The Lancet. 2005; 366: 2112-2117. 79. Wimo et al. Cost of illness and burden of dementia – The base option, 2009. Available at http://www.alzheimer-europe.org/FR/Research/ European-Collaboration-on-Dementia/Cost-of-dementia/Cost-of-illness-and-burden-of-dementia 80. Pfizer. Pipeline as of November 2012. Available at http://www.pfizer.com/research/product_pipeline/product_pipeline.jsp. 81. WHO. Bacterial Infections Staphylococcal Infection webpage. Available at http://www.who.int/vaccine_research/diseases/soa_bacterial/en/ index2.html 82. WHO. Meningococcal Meningitis Fact sheet No. 141, December 2010. Available at http://www.who.int/mediacentre/factsheets/fs141/en/ 83. CDC. Meningitis in Other Countries webpage. Available at http://www.cdc.gov/meningitis/global.html. 84. CDC. Clostridium Difficile Information for Patients webpage. Available at http://www.cdc.gov/hai/organisms/cdiff/Cdiff-patient.html. VAC/2013/001/1 Date of preparation: April 2013.