* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download immunotherapy in allergic diseases
Survey
Document related concepts
Transcript
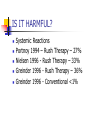
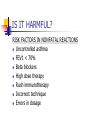
IMMUNOTHERAPY IN ALLERGIC DISEASES Dr Narayan Pradeep Pulmonologist kasaragod IMMUNOTHERAPY IN ALLERGIC DISEASES History Definition Is it useful? Is it harmful? Mechanisms of action Indications Contraindications IMMUNOTHERAPY IN ALLERGIC DISEASES Guidelines for immunotherapy Precautions Types of Immunotherapy Effects of withdrawal Case studies History 1565 – 1819 – 1872 – 1873 – Leonardo Botello Seasonal Allergy Bostock Classical Case of Hay Fever Morrill Wyman Autumnal Catarrh Blackley Grass Pollen Counts History 1900 - 1910 – 1913 – Curtis Aqueous extracts for immunization Leonard Noon Subcutaneous injections of pollen extracts Standardisation by wt Clowes Demonstrated a 1000 fold increase in resistance by conjunctival testing History 1914 - Robert Cooke Basics of Immunotherapy as practiced today PNU – Kjeldahl method Mechanisms of Immunotherapy Blocking antibodies Dosage and testing techniques History 1968 – Norman Immunotherapy replace Desensitization 1980’s – Newer routes of drug delivery Sublingual Immunotherapy 1990’s – Oral microencapsulated Local Nasal, Bronchial History 1998 – 60 million patients annually treated in the world 33 million injections every year in USA alone 2002 - 50% of Immunotherapy in Europe is Sublingual Definition Allergy An exaggerated response on exposure to allergen following prior exposure, mediated by an immune reaction involving IgE Definition Atopy Increased tendency to IgE based sensitivity to common environmental allergens in genetically predisposed patients Definition Immunotherapy The technique of treating IgE mediated disease with increasing doses of an allergen in order to decrease sensitivity to that allergen IS IT USEFUL? Large Volume of data in favour Van Metre ( 1980) Decrease in symptoms Decrease in medication use Improvement limited to antigens used IS IT USEFUL? Horak (1993) Immunotherapy is an integral component in treatment strategy Standardized extracts improve treatment safety and efficacy IS IT USEFUL? British Society Position Paper – 1993 Immunotherapy reduces inflammation and bronchial hyper responsiveness European Academy of Allergy & Immunology – 1993 Immunotherapy influences favourably the progression of clinical disease IS IT USEFUL? Canadian Guidelines (1995) Immunotherapy is effective in patients with allergy to insect stings, allergic rhino conjunctivitis and in some patients with asthma who have been correctly diagnosed through a cautious history and corroborated with positive skin test results IS IT USEFUL? Donovan (1996) Schoen wetter (1996) Nasal symptoms significantly less Correct allergen, adequate dose, appropriate patient, safe and effective Creticos ( 1996) Decreases hay fever symptoms, skin sensitivity & sensitivity to bronchial challenge IS IT USEFUL? New Zealand, Australia and Australasian Society on Allergy – 1997 Immunotherapy should not be regarded as an alternative to established forms of preventive therapy Safe and Effective IS IT USEFUL? Adkinson (1997) Children with moderate to severe perennial asthma Not much benefit Small number of patients Too many aeroallergens Timothy Craig(1998) Data to support use is less concrete IS IT USEFUL? META ANALYSIS ABRAMSON – 1995 – Adults ROSS – 2000 - Adults 20 Randomized Placebo Controlled Double Blind trials between 1966-80 Concluded that Immunotherapy is effective TO OVERTURN THESE RESULTS 33 NEGATIVE STUDIES ARE REQUIRED IS IT USEFUL? META ANALYSIS ABRAMSON – 1995 – Adults ROSS – 2000 - Adults Reductions in symptoms Decreased need for asthma medications Improved lung functions Decreased bronchial hyper reactivity IS IT USEFUL? META ANALYSIS Arnaldo (1998) Children 27/29 studies of controlled pediatric studies with 1443 children aged 2-14 Beneficial effects on Natural History Some had total remissions Needed to complete immunotherapy CONCLUDED ONLY CURATIVE TREATMENT FOR ASTHMA, SAFE IS IT USEFUL? PREVENTION OF ASTHMA PREVENTING DISEASE PROGRESSION Preventive immunotherapy – Jacobsen 2001 Prevention of Asthma Treatment – PAT study - 2002 IS IT HARMFUL? Side Effects Usually minor Timothy Craig 1998 Local Swelling, redness in 15% British Society of Adverse events 1993 1/500 injections IS IT HARMFUL? Hejjaoui 1992, Wells 1996 Risk increases with Rush Desensitization Use of high doses Uncontrolled asthma Strongly positive skin tests Change of vials Prior Anaphylaxis IS IT HARMFUL? Systemic Reactions Portnoy 1994 – Rush Therapy – 27% Nielsen 1996 - Rush Therapy – 33% Greinder 1996 - Rush Therapy – 36% Greinder 1996 - Conventional <1% IS IT HARMFUL? Committee on safety of medicines – BMJ 1986 1/ 27,854 injections In 29 years, 14,59,273 courses of treatment – 29 deaths Lockey and Reid 1993 24 deaths from 1959-1984 17 deaths from 1985 –1989 ERRORS OF TREATMENT IS IT HARMFUL? RISK FACTORS IN NONFATAL REACTIONS Uncontrolled asthma FEV1 < 70% Beta blockers High dose therapy Rush immunotherapy Incorrect technique Errors in dosage IS IT HARMFUL? RISK FACTORS IN FATAL REACTIONS Symptomatic asthma High degree of allergen sensitivity Injections during seasonal exacerbation Injections from new vials Errors in dosage Beta blockers MECHANISMS OF ACTION IMMUNOGLOBULINS Creticos(1984), Arnoldo (1998), Lu Fm (1998), Patterson ( 1998) IgE - Levels initially rise, then falls IgG - Increases many folds, 8.8 fold rise IgG4 - Specific to the antigen increases MECHANISMS OF ACTION BIOCHEMISTRY Creticos(1989) Decreased Histamine Decreased tosyl - L - arginine - methyl esterase(TAME) - activates kinin system Decreased Prostaglandins MECHANISMS OF ACTION MAST CELLS Creticos(1989) Obrein (1997) Decreased release of mast cell mediators MECHANISMS OF ACTION EOSINOPHILS Van Bever (1990) Ohasi(1997) Decreased Eosinophil recruitment and influx Decreased Eosinophil activation Decreased Eosinophil Cationic protein MECHANISMS OF ACTION T LYMPHOCYTES & CYTOKINES Roy Patterson (1998) Obrein (1997) Lu Fi(1998) Change of CD4 cells from Th2 to Th1 phenotype (2002) Down regulation of IL- 4 production from T cells MECHANISMS OF ACTION SUMMARY Stephen Durham (1998) Reduces early phase reaction Reduces late phase reaction Reduces concentration of inflammatory mediators Reduces nasal mast cells Reduces eosinophils, eosinophil cationic protein MECHANISMS OF ACTION SUMMARY Modify T Lymphocyte response Increases gamma interferon, IL 12, IL2 Not known whether immune deviation due to anergy of Th2/ Th0 or increase of Th1 Amplification of CD8 cells downregulatory INDICATIONS IgE mediated disease Skin test or RAST positive for a specific antigen Correlation between allergic symptoms and test results No relief of symptoms with environmental changes or not possible to avoid exposure INDICATIONS Failure to obtain relief with medications Failure to tolerate medications Unwillingness to take long term medications Significant allergic upper airway or ocular disease strengthens indication INDICATIONS IN PREGNANT PATIENTS Immunotherapy should not be initiated during pregnancy Immunotherapy can be continued in pregnancy if she has been tolerating it well No teratogenesis observed IMMUNOTHERAPY IN ASTHMA GINA I Asthma GINA II Asthma GINA III Asthma Pharmacotherapy Immunotherapy GINA IV Asthma IMMUNOTHERAPY IN RHINITIS Mild Rhintis & Conj Severe Rhintis & Conj Moderate Rhinitis & Conj Pharmacotherapy IMMUNOTHERAPY CONTRAINDICATIONS ABSOLUTE Concomitant use of Beta Blockers Risk of Anaphylaxis Responds poorly to Resuscitation Previous Anaphylactic reaction to Immunotherapy Lack of adequate resuscitation facilities Clinicians without training CONTRAINDICATIONS RELATIVE If FEV1/PEFR < 70% predicted Unstable Asthma Nocturnal Asthma Use of bronchodilator more than thrice a week Diurnal variation >20% Bronchodilator reversibility >20% CONTRAINDICATIONS RELATIVE Autoimmune disease or Malignancy Pregnancy – do not initiate Bronchospasm to previous injection Children <5 years of age Eczema – may flare up Beta Blocker eye drops Unstable coronary artery disease GUIDELINES FOR IMMUNOTHERAPY AAAAI & CSAI Should not be self administered Physician’s office, emergency equipment available Subcutaneous High potency extracts needed Appropriate dose reductions made in delays, vial change, reactions GUIDELINES FOR IMMUNOTHERAPY AAAAI & CSAI Informed consent Universal Precautions Individual dosage schedule Store extracts at 4 degree centigrade Clinical & Peak flow before & after Inj Stays at clinic for at least 30 minutes Strenuous exercise, hot baths after 6 hr GUIDELINES FOR IMMUNOTHERAPY AAAAI & CSAI Local swelling > 50mm requires dosage reduction No relief for 2 years- discontinue High dose therapy usually for 5 years PRECAUTIONS Administer cautiously in all asthmatics PFT/ Peak flow >70% PFT/ Peak flow before patient leaves clinic Stick to dosage schedule Irregular patients at risk of reactions If schedule needs to change - Allergist PRECAUTIONS All vials to be properly labelled Check patients name and number and vial and dosage at each visit Even after reaching maintenance – decrease dose when new vial is started Fever, acute asthma skip the injection TYPES OF IMMUNOTHERAPY SUBCUTANEOUS INJECTIONS – 90 YRS Conventional Months to maintenance Rush Days/ weeks for maintenance Rapid relief & good compliance Hospital admission High systemic reactions TYPES OF IMMUNOTHERAPY Short term Seven preseasonal injections Zenner EXTRACTS Specific Monotherapy Specific Mixture Nonspecific Mixture TYPES OF IMMUNOTHERAPY NEW METHODS Sublingual - 25 years Intranasal aqueous Nelson(1993) Chando(1995) Andri(1995) Intranasal powder Andri (1996) TYPES OF IMMUNOTHERAPY Oral Oral encapsulated Bordignon ( 1994) Litwin (1997) Van Densen(1997) Local bronchial Bjorksten (1994) EFFECTS OF WITHDRAWAL Medin G (1995) Beneficial effects persists for years after full course of IT Studies demonstrated both long term and short term benefits Trial of discontinuation attempted after 5 years EFFECTS OF WITHDRAWAL Donovan (1997) Initial IT significantly reduced symptoms On stopping IT partial return of mediators, decline in IgG noted Seasonal increase in IgE did not occur Symptom improvement persisted Sublingual immunotherapy RCT have now demonstrated the efficacy of sublingual immunotherapy WHO approved this modality After nearly 10 years of starting the procedure in Europe the procedure picked up in USA and UK in few centres Sublingual Immunotherapy Indications initially were Patients who cannot tolerate injections Children who are very apprehensive about injections Absorption and mechanism Sublingual immunotherapy uses the principle of oral absorption Easily reaches submandibular lymph channels Faster than subcutaneous route (absorption) Advantages > 50% Allergologist switched over to sublingual immunotherapy Rarely produces any reactions More compliant to therapy Children can also take Disadvantages Side effects are extremely rare Mouth itching and soreness of throat Sneezing Increase in asthma symptoms initially ( yet to report, only theoritical ) Duration Higher the dose earlier the effect 2 to 3 years on an average Can be continued upto 5 years In Nearly 70 to 80 % the treatment can be stopped much earlier CASE STUDY - 1 Mrs. JH 40 years is suffering from asthma for the past 20 years and needs medicine daily for her asthma(GINA III) Her daily requirement to be symptom free - inhaled corticosteroids 1000 microgram of Fluticasone with LABA. She has recently developed joint pains and swellings and has early morning stiffness. CASE STUDY - 1 What are the investigations planned for her allergy? Is GINA III Asthma an indication for immunotherapy? Will you give her immunotherapy? CASE STUDY - 1 What are the investigations planned for her allergy? – Allergy Test or Specific IgE Is GINA III Asthma an indication for immunotherapy? - Yes Will you give her immunotherapy? Investigate for Rheumatoid arthritis – Collagen Vascular disease is a Contraindication Thank u For patient hearing