* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Notes on Chemistry - Properties of Atoms
Survey
Document related concepts
Transcript
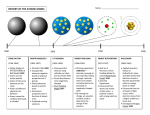
+ Atoms, Ions, and Isotopes Oh my! + http://www.projectsharetexas.org/sites/default/files/resourc es/documents/EvolutionOfAtomicModel.pdf + Name that component! Has a negative charge Has a positive charge Has no charge Has a relative mass (to proton) of 1 Has an actual mass of 1.67x10-24 Has an actual mass of 9.11 x 10-28 + Objectives for Today Characterize protons, neutrons, electrons by location, relative charge, relative mass (p=1, n=1, e=1/2000). • Use symbols: A= mass number, Z=atomic number • Use notation for writing isotope symbols:235 92 or U-235 • Identify isotope using mass number and atomic number and relate to number of protons, neutrons and electrons. • Differentiate average atomic mass of an element from the actual isotopic mass and mass number of specific isotopes. (Use example calculations to determine average atomic mass of atoms from relative abundance and actual isotopic mass to develop understanding) + How will we do that? Group read and presentation Everyone takes notes on presentation I recap anything necessary Significant Figures and Scientific Notation Introduce Electron shells Discuss Uranium and the nuclear bomb + Elements on the periodic table + Elements Distinguished Atomic number correlates with number of protons Therefore an atom is defined by its number of protons Therefore an atom is unique by its positive charged elements Have a variable mass Number of neutrons can change (will get into this more later) Have by atomic number a variable charge Number of neutrons can change (ibid.) + Calculating things with elements The number of neutrons in an atom is the difference between the mass number and atomic number # of Neutrons = Mass # - atomic # + Isotopes Atoms with the same number of protons but different number of neutrons Therefore, mass changes, charge changes Hydrogen isotopes Hydrogen – 1 (Hydrogen) Hydrogen – 2 (deuterium) Hydrogen – 3 (tritium) What happens when we add a fourth neutron? + Atomic Mass Units Reference Isotope is Carbon – 12 Has six protons, six neutrons and one amu is equal to Mass of carbon-12 divided by 12 (presumably an approximation of the mass of a proton or neutron) + Overall amu To calculate the atomic mass of an element, multiply the mass of each isotope by its natural abundance, expressed as a decimal and then add the products + Groups Difference between atomic number and mass number Isotopes Atomic mass unit and natural abundance Preview of periodic table + Catalyst 9/16 Collect one of each handout on table Hole punch them if you need to Then be seated by the time the bell rings. + Objectives Understand what an ion is Analyze diagrams related to the Bohr model of the hydrogen atom in terms of allowed, discrete energy levels in the emission spectrum. • Describe the electron cloud of the atom in terms of a probability model. • Relate the electron configurations of atoms to the Bohr and electron cloud models. Become familiar with the math of science + To accomplish these we will Take some notes Do a demonstration Complete several work sheets on the science of math + https://www.youtube.com/watch?v=WWc3k2723IM https://www.youtube.com/watch?v=thnDxFdkzZs Then Carbon as an example + Bohr Model + + Probability Model Describes the areas around the nucleus in which we are likely to find the electrons, depending on their energy level and element. + + Activity + Catalyst 9/17 Review: Name the properties (relative mass, charge, location) of an electron, proton, and neutron What happens when the number of protons changes? Neutrons? Electrons? The atomic mass listed on the periodic table is the average atomic mass. In order to calculate it, we need to know what isotopes exist, and their natural abundance. Use words or a formula to state how we calculate average atomic mass What are some difference between the Bohr Model and the Electron Cloud (Probability Model)? + But first, let me talk about ions https://www.youtube.com/watch?v=WWc3k2723IM Don’t overthink this: 3+ Se refers to an ion with a positive 3 charge Therefore is missing 3 electrons + Electron Configurations Bohr model indicates seven energy levels Electron Cloud/Probability Model indicates seven levels, but up to (and maybe more) four sublevels These are s, p, d, f They look pretty crazy You need to know 3 principles of calculating Aufbau Principle Pauli Exclusion Principle Hund’s Rule + Aufbau Electrons fill lowest available energy shells first S then p then d then f, until the third orbital 3 4s happens before 3d + Pauli Exclusion Principle Each orbital can contain two electrons Each electron has a positive or negative spin These are written as and Pauli Exclusion Principle Electrons first fill every available empty orbital before taking a negative spin next to a positive electron + Quanta A unit of energy When energy is applied to an atom, the electrons move from a lower/ground state to an excited state When energy is released from the atom, an electron goes back down to ground state and the energy takes the form of EM wave discharge + Significant Figures http://www.usca.edu/chemistry/genchem/sigfig.htm + Catalyst 9/24 Please take one sheet of paper. Keep it face down. We’ll get to it later There are a number of different articles; yours may not be the same as your neighbors. That’s fine. Actually, it’s not that fine. You shouldn’t know what your neighbor has because you shouldn’t have turned it over. After announcements we’ll have a few test questions to go over. Have your note sheets ready. Turn the packet in to the bin at the front. + + What pieces of information would help you answer this question? Write down any strategies you can use to answer the question. + + Objectives Articulate that this electromagnetic radiation is given off as photons. • Understand the inverse relationship between wavelength and frequency, and the direct relationship between energy and frequency. • Use the “Bohr Model for Hydrogen Atom” and “Electromagnetic Spectrum” diagrams from the Reference Tables to relate color, frequency, and wavelength of the light emitted to the energy of the photon. • Explain that Niles Bohr produced a model of the hydrogen atom based on experimental observations. This model indicated that: 1. an electron circles the nucleus only in fixed energy ranges called orbits; 2. an electron can neither gain or lose energy inside this orbit, but could move up or down to another orbit; 3. that the lowest energy orbit is closest to the nucleus. • Describe the wave/particle duality of electrons. + Homework Finish any homework or assignments that you need to finish Turn these in tomorrow morning!!!!!!!!!!!!!!! Stay tuned for a wardrobe request + + On light, and color http://web.archive.org/lessons/how-do-we-see-color-colmkelleher#review + Before reading… Group A: List everything you know about particles Group B: List everything you know about waves Group C: List everything you know about light Read and annotate the article What evidence does the author present? What are some things the author doesn’t consider? + Next: Groups get together: collect and refine evidence for your view of the electron Each group presents their view of the electron + http://phys.org/news/2015-03-particle.html + Homework: Group A: wear green Group B: wear blue Group C: wear red This is for bonus points on your next quiz + Catalyst 9/25 Take out the worksheets related to “Electron Configuration” that I gave last week. Turn in any overdue homework All this is done silently Start looking over all the questions. Start by checking off which ones you feel you cannot answer. Circle those, and on the index card, write what information you’ll need to solve them. Then answer the ones you feel you know. + Objectives/Plan for today Understand electron configuration as modeled by the Bohr and Electron Cloud model Understand light Prepare for review for Tuesday Quiz + Catalyst 9-28 Write out the Orbital Notation and Electron Configuration for Iodine Iodine-127 is the most stable and common isotope for the element. What is the atomic mass of Iodine-127? How many protons are in Iodine 127? How many neutrons? Given that the Periodic Table shows Iodine with an atomic mass of 126.90, what does this say about the natural abundance of Iodine 127? + Objectives Understand Bloom’s Revised Taxonomy Review material from this objective Create study guides for further review Prepare for quiz tomorrow + Bloom’s Taxonomy applied + Your study guide Must address all objectives Can be no more and no less than 12 questions One question should be an exploration of a certain element, as demonstrated in the catalyst This element must be in the fourth period or below Should have a question that applies each of the bottom four levels of Bloom’s Revised Taxonomy Bonus points for each additional objective covered in the same question and each additional level of thinking (Bloom) Group with the most points gets a bonus point on the quiz