* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Population Pharmacokinetics
Plateau principle wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Orphan drug wikipedia , lookup
Drug design wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Neuropharmacology wikipedia , lookup
Compounding wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Clinical trial wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
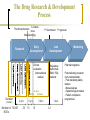
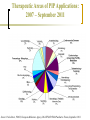
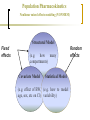
Clinical Research in Children and the Role of Population Pharmacokinetics Analyses Univerzitetska dečja klinika Beograd Gregory B. Sivolapenko Associate Professor Laboratory of Pharmacokinetics, Director University of Patras, Patras, Greece DANI UNIVERZITETSKE DEČJE KLINIKE Mesto održavanja: Sava centar, Beograd 09.-10.12.2011 The Drug Research & Development Process st Preclinical decision 1 human dose Manufacturing 1st Submission 1st Approval Early Development Research Clinical evaluation (interventional studies) (median) Number of 10,000 NCEs Regulatory Authorities: EMA, FDA, national In vivo animals humans In vitro Duration Late Development 4.2yrs 33 1.3yrs 13 5.8yrs 10 1.4yrs 1-2 Marketing Pharmacovigilance Post-marketing research (non-interventional): - Post-marketing safety studies - Meta-analyses - Epidemiological studies - Patient compliance programmes The Drug Research & Development in Children Most prescription drugs lack the information and the appropriate pharmaceutical formulation to support their use in children. Without the provision of appropriate information concerning paediatric dosing, safety or efficacy, physicians who treat children must decide between withholding treatment proven effective in older patients or participating in the practice of off-label use by prescribing to children products not studied for paediatrics. The use of unlicensed and off-label medicines in children is widespread. 2 surveys in the Physicians Desk Reference showed that while 78% of products lacked sufficient information or labeling regarding paediatric use in 1973, this had increased to 81% in 19991,2 In the EU, >50% of medicines used in children have never been actually studies in this population, but only in adults, and not necessarily in the same indication or the same disease3. 1. 2. 3. Source: Gilman JT and Gal P. Clin. Pharmacokinet., 1992; 23 (1): 1-9 Source: Wilson JT. Paediatrics, 1999; 104 (3): 585-590 Source: Conroy et al. Br. Med. J.; 2000, 320: 79-82 The Drug Research & Development in Children Problems resulting from the absence of suitably adapted medicinal products for the paediatric population include: Inadequate dosage information which leads to increased risk of side-effects Ineffective treatment through under-dosage No availability to the paediatric population of therapeutic advances, suitable formulations and routes of administration Market forces alone have proven insufficient to stimulate adequate research into, and the development and authorisation of medicinal products for the paediatric population, mainly due to: Small market size Need for development of paediatric formulation(s) Practical and ethical challenges in paediatric clinical research 5 subgroups in paediatric population: Regulatory Legislations for Paediatric Drug Development Over the past decade, regulatory legislations for drug development in paediatric patients were passed worldwide, dramatically increasing the number of drugs tested in and labeled for children. Both the USA FDA and the EU EMA established approaches that have been successful in generating important new information about the safety and efficacy of drugs used by children. Today, the need for more studies to obtain paediatric information for medicines used in children becomes a matter of consensus on a global basis. However, this should be done without compromising the well-being of paediatric patients participating in clinical studies. Regulatory Aspects of Paediatric Drug Development: the US perspective 1994 Pediatric Labeling Rule: Voluntary. Required drug manufacturer to survey existing data and extrapolate efficacy from adults, supplementing with paediatric PK studies. Few studies done. 1997 Pediatric Rule: Required the drug manufacturer of a new drug to submit before approval, safety and effectiveness information in relevant paediatric age groups for the claimed indications. 1997 FDA MA: Introduced a process in which the FDA developed a list of drugs for which additional paediatric information might be beneficial, issuing a Written Request (WR) to the drug manufacturer for paediatric studies. If companies submitted studies in response to a WR, 6 months additional market exclusivity were granted. 2002 BPCA: Renewed the 6-month exclusivity incentives, created a process for on and off-patent drugs (involving government contracts), and mandated public disclosure of study results. 2003 PREA: Put into legislation most components of the 1994 Pediatric Rule. Required paediatric assessment for certain applications, a paediatric plan and the development of age-appropriate formulation. 2007 FDA AA: Reauthorised and extended BPCA and PREA until October 2012. Introduced the Pediatric Review Committee (PeRC). Regulatory Aspects of Paediatric Drug Development: the US perspective As of the end of August 2010, the FDA received 610 Proposed Paediatric Study Requests, and issued 394 Written Requests. As of the end of August 2010, the FDA granted paediatric exclusivity for 173 approved drugs Between September 2007 and June 2010, 273 studies were completed under the BPCA and/or the PREA under the FDA AA. As of the end of August 2010, the total number of products under BPCA and PREA were 38 and 65 respectively. Statistics published at: FDA web page “Pediatric Exclusivity Statistics” Regulatory Aspects of Paediatric Drug Development: the EU perspective 1997 EC Round Table: Experts discussed paediatric medicines at the EMA. 1998: The Commission supported the need for international discussion on the conduct of cinical trials in children in the context of the International Conference of Harmonisation (ICH). 2000 ICH Guideline E11: “Clinical investigation of medicinal products in the paediatric population” became a European guideline. 2002 Consultation Paper: The European Helath Council requested the Commission to take specific action to remedy the problem of usage of unauthorised medicinal products in children. Commission produced the Consultation Paper “Better medicines for children – proposed regulatory actions on paediatric medicinal products”. 2006: Paediatric Regulation agreed. 2007: Paediatric Regulation 1901/2006 and its amendment 1902/2006 entered into force. For products already on the market for use in adults, that are not covered by a patent or a supplementary protection certificate, the regulation created a special application mechanism, the Paediatric Use Marketing Authorisation (PUMA). A PUMA may contain data from new clinical studies, data from pediatric studies in the literature or data from studies in dossiers of other approved products. This mechanism was intended to provide an incentive for small and mid-size companies to develop products for use in children. The regulation provides 10 years of market protection as a reward for conducting clinical trials in paediatric patients for products approved under a PUMA. EU Paediatric Regulation EU 1901/2006 Paediatric Regulation The paediatric regulation aims to: facilitate the development and accessibility of medicinal products for children ensure that medicinal products used to treat paediatric population are subject to ethical research of high quality ensure that medicines for children are appropriately authorised improve the information available on the use of medicinal products in the various paediatric populations The above objectives should be achieved without subjecting the paediatric population to unnecessary clinical trials and without delaying the authorisation of medicinal products for other age populations. EU 1901/2006 Paediatric Regulation No marketing authorisation is granted for new products without compliance with an agreed Paediatric Investigation Plan (PIP) The PIP is agreed with the Paediatric Committe (PDCO) of the EMA and is binding to the applicant. The PDCO was established within the EMA by July 26th, 2007. The PDCO is composed of: 5 members from the CHMP 1 member by each EU member state 3 members representing health professionals (appointed by the EC) 3 members representing patients’ associations (appointed by the EC) Between August 2007 and December 2009, the PDCO received 629 validated PIP applications (961 indications, average 1.5 indication per product). Report to EC, April 27, 2010, EMA, Section Paediatric Medicines. Approximately 700 PIPs have been agreed up today (EMA Workshop 29-30 November, 2011). Estimated >300 new paediatric trials will be initiated. EEA 2005-2010: the number of paediatric studies have increased from ~600 studies to 949 studies 2005 2006 2007 2008 2009 Source: Paolo Tomasi, Head of Paediatric Medicines, European Medicines Agency, March 2011 2010 Therapeutic Areas of PIP Applications: 2007 – September 2011 Source: Paolo Rossi, PDCO, European Medicines Agency, DIA/EFGCP/EMA Paediatric Forum, September 2011 Enpr-EMA Enpr-EMA: May 2010, the EMA with the scientific support of the PDCO, developed a European network of existing national and international networks, investigators and centres, with specific expertise in the performance of studies in the paediatric population. Objective: to coordinate studies related to paediatric medicinal products, to build up competence at a European level, to avoid unnecessary duplication of studies and testing in the paediatric population. Operational goals: To link together existing networks To provide infrastructure for industry to conduct studies in children To define consistent and transparent quality standards within the European network To harmonise clinical trial procedures To define strategies for resolving major challenges To communicate with external stakeholders Recognition criteria for membership EMA Meetings on Paediatric Clinical Research The EMA regularly organises and invites expression of interest for workshops Workshop on Ethics of Clinical Trials in Children, 29 & 30 November 2011, London, UK Joint DIA/EFGCP/EMA Paediatric Forum: the paediatric regulation in its 5th year, 26 & 27 September 2011, London, UK 3rd Workshop on Network of Paediatric Research, Enpr-EMA, 10 & 11 March 2011 Implementing Clinical Trials in Children Drug development for paediatric patients is accompanied by various challenges. Clinical studies in children are different from studies in adults, and the planning and conduct of a paediatric study needs special attention since the patient population is more vulnerable. Companies performing the paediatric studies as per the agreed PIP, frequently encounter problems during the review by the Ethics Committees. Implementing Clinical Trials in Children Practical and ethical considerations are prominent. The main challenges are: Relative small number of paediatric patients Consent and assent Defining the first dose in children Sampling strategies Right methods for data collection and analysis Generate knowledge about safety, efficacy, pharmacokinetics and pharmacodynamics Determine the right dose and dosing regimen Paediatric formulations Dosages according to weight? Hydration of skin? Oral dosing, mixing with food? Parenteral, volume, needle Presrervatives, colourants, sweeteners Taste or smell? Experience with Ethical Review of Paediatric Trials in the EU The EUCROF Survey: September to October 2011 Concerns from Ethics Committees: Child Protection Change of IC, assent per age group Burden of participants, impact on schooling Exclusion of mentally disabled minors Study Procedure Blood volume collection, number of vena punctures Study Design Benefit of study to paediatric subject Clarifications of the size sample calculations Use of placebo Others Qualification of investigator and paediatric experience Insurance coverage Modeling & Simulation in Clinical Research To comply with regulatory requirements but also to optimise paediatric drug development, in terms of ethical, operational and scientific aspects, new and appropriate strategies for clinical studies in children need to be developed. Computerised modeling and simulation techniques are beneficial tools for optimisation of the design, increasing the knowledge gained from paediatric studies. Population Pharmacokinetics The study of the sources and correlates of variability in drug concentrations among individuals of a target population when clinically relevant doses of the drug are administered. Advantages Analysis of sparse and unbalanced data Conduct of PK studies in special populations Estimation and explanation of variability Development of individualized dosing regimens Minimisation of participant number with reduced undue risk, distress, pain and fear Disadvantages Complex methodology: mathematics, statistics, software packages Population Pharmacokinetics Patient 1 Patient 2 Traditional approach Concentration (ug/L) 10000.00 1000.00 100.00 10.00 1.00 0 1 2 3 4 Time (hrs) 5 6 Patient 1 Patient 2 Population approach Concentration (ug/L) 10000.00 1000.00 100.00 10.00 1.00 0 1 2 3 4 Time (hrs) 5 6 Population Pharmacokinetics: analysis methods Naive pooled data analysis Standard two-stage approach Bayesian two-stage approach Nonlinear mixed effects modeling (NONMEM): Widespread application in paediatric PK studies Simultaneous analysis of all data of the study population without ignoring the individual profiles Analysis of sparse and unbalanced data Samples taken during routine treatment can be used Relationships between covariates and parameters are explored Inter-, intra-individual and inter-occasion variability of the parameters can be estimated Drug-specific and biological system-specific properties can be identified Population Pharmacokinetics Nonlinear mixed effects modeling (NONMEM) Structural Model Fixed effects (e.g. how many compartments) Covariate Model Statistical Model (e.g. effect of BW, (e.g. how to model age, sex, etc on Cl) variability) Random effects Population Pharmacokinetics Cj =D/Vdj*e(Clj/Vdj )*tj + εj Source: Vozeh S. et al. Eur J Clin Pharmacol 1982;23:445-451 Population Pharmacokinetics in Children Rapid maturation of organ functions important for drug absorption, distribution and elimination is a specific feature of very young paediatric patients, therefore changes in dose may be necessary for a patient over time, based on individual maturation. Pharmacokinetic studies in the paediatric population reveal which dose will produce blood levels similar to those observed in adults. In this way, adults’ efficacy data may be extrapolated to the paediatric population, provided that: The disease process is similar in adults and children and the outcome of therapy is likely to be comparable The drug’s effect is well characterised with regard to important PK parameters, and these parameters have been well correlated with activity in adults Population Pharmacokinetics Total body elimination rate constant (k) and total body Clearance (Cl) are considered to be the main drivers of differences in pharmacological drug response in the paediatric population. Covariate models which describe the developmental changes of clearance pathways in paediatric population pharmacokinetic models are crucial to determine the first-in-child or evidence-based dosing regimen, as this covariate relationship describing these changes can be directly used in drug dosing algorithms. A population PK model for morphine and its glucuronides in children Morphine: metabolic pathway through glucuronidation by uridine diphosphate glucuronosyltransferase (UGT) 2B7 Question: if age (maturation) influences the capacity of glucuronidation Patients: 248 children preterm and term neonates, infants and children <3 y Dosing: Loading dose= 100 μg/kg Maintenance dose= 10 μg/kg/h (infusion) Source: Knibbe C.A.J. et al. Clin Pharmacokinet 2009; 48(6):371-385 A population PK model for morphine and its glucuronides in children V1*BW (M) Qeq V2*BW (M) Cl1*BW1.44 Cl2*BW1.44 V3*BW (M3G) V4*BW (M6G) Cl3*BW1.44 Cl4*BW1.44 The influence of BW on the formation and elimination clearances of the drug’s glucuronides was best described by an allometric equation with an exponential scaling factor of 1.44. Postnatal age (PNA) <10 d. was an additional covariate for Cl1 and Cl2 (slower Cl in newborns <10 d.) ------Population prediction in children <10 d. Population prediction in children >10 d. Individual prediction in children <10 d. Individual prediction in children >10 d. A population PK model for morphine and its glucuronides in children Model-based simulations showed that a narrower range of morphine 10 μg/kg/h and when a maintenance dose of 10 μg/kg1.44/h instead of 10 μg/kg/h is administered 50% reduction of maintenance dose is required in neonates <10 d due to reduced glucuronidation capacity Morphine concentration (ng/mL) metabolite concentrations is achieved 10 μg/kg1.44/h and 50% <10d. A linear equation was found to describe the influence of BW on the volumes of distribution ------ children <10 d. children >10 d Time (min) Extrapolation of findings on UGT maturation It is reasonable to assume that that the results with morphine can be extrapolated to all drugs metabolized by the same (iso)enzyme. Zidovudine is also metabolized by UGT 2B7 It was found1 that the morphine covariate model could also describe Zidovudine glucuronidation: Cln = Clmedian * BW1.44 Population models that describe the maturation of specific elimination pathways are system specific properties (drug independent). Maturation models can be applicable to drugs that are eliminated through the same pathway expediting the development of pediatric population models. Propofol, UGT 1A8/9 1. Source: Krekels E.H.J. et al. Semi-physiological model for glucuronidation in neonates and infantsapplication to zidovudine. Br J Pharmacol 2011; in press Regulatory Authorities and Population Pharmacokinetics Analyses Several regulatory guidance documents refer explicitly to computerised modeling and simulation methodology, therefore today there is a clear regulatory basis (and a need) for using modeling and simulation in paediatric drug development: “Guidance for Industry: population pharmacokinetics”, FDA, CDER/CBER, Febr. 1999 “Guidance for Industry: pharmacokinetics in patients with impaired hepatic function. Study design, data analysis and impact on dosing and labeling”, FDA, CDER, Clinical Pharmacology, May 2003 “Guidance for Industry: pharmacokinetics in pregnancy. Study design, data analysis and impact on dosing and labeling” FDA, CDER, Clinical Pharmacology, October 2004 “Guideline on the role of pharmacokinetics in the development of medicinal products in paediatric population”, EMEA, CHMP/EWP/147013/04corr., 28 June 2006 “Guideline on reporting the results CHMP/EWP/185990/06, 21 June 2007 of population pharmacokinetic analyses”, EMEA, “Reflection paper on the use of pharmacogenetics in the pharmacokinetic evaluation of medicinal products” EMEA, 128517/06, 25 May 2007 The Application of Population Pharmacokinetic Analyses in the Drug Indications Examples (US PDR): Humira (adalimumab, Abbott), arthritis, + age ReFacto (antihaemophilic factor VIII, Wyeth), haemorrhagic episodes, gen PK Avastin (bevacizumab, Genentech/Roche), met. Ca-colorectal, gen PK Erbitux (cetuximab, Merck), Ca-colorectal, + sex Ciproxin (ciprofloxacin, Bayer), antibacterial, - age Taxotere (docetaxel, Aventis), Ca-breast, NSCLC, Ca-prostate, gen PK Enbrel (etanercept, Amgen/Wyeth), arthritis, - age Neurontin (gabapentin, Pfizer), epilepsy, post-herpetic neuralgia, + age Gemzar (gemcitabine, Lilly), Ca-breast, NSCLC, Ca-pancreatic, gen PK,+sex/age Gleevec (imatinib, Novartis), CML, GIST, gen PK Zofron (ondansentron, GSK), prevention of nausea and vomiting, gen PK, - age Pegasys (IFN-a2a, Roche), hepatitis C, + age Viagra (sildenafil, Pfizer), erectile dysfunction, - alc Risperdal (risperidone, Janssen-Cilag), schizophrenia, - sex, - race Source: J.Z. Duan, FDA, J.Clin.Pharm.Therapeutics, 32: 57-79, 2007 Conclusions Drug Research and Development is still an expensive and long process. Relatively less research had been done in paediatruc populations. A large number of drugs used in children do not possess adequate information related to the safe and effective dosing scheme. Recent developments in US and EU regulations, augmented paediatric clinical research, by voluntarily and/or compulsory measurements. US FDA and EU EMA strengthened the flow of information and facilitated reportings in relation to paediatric clinical trials, in order to avoid unnecessary repetitions. The recent EU Regulation 1235/2010 and Directive 2010/84/EU on Pharmacovigilance, will enhance Post Marketing Safety Studies. Conclusions It is anticipated that in the foreseeable future more drugs will be used on-label by children, despite the difficulties in implementing a paediatric clinical trial. Concerns exist regarding the adequacy of paediatric research in younger age groups (infants, toddlers, younger children) and the advances in paediatric pharmaceutical formulations. Concerns about the overall increase of cost of paediatric medicines. Our growing knowledge on pharmacogenetics and pharmacogenomics will result in more and more detailed paediatric clinical studies. Population pharmacokinetic studies is a tool for dose adjustments in any paediatric sub population, with the least discomfort for the children, providing information on the optimal safe, and effective dosing scheme.