* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Structures of some Juvenile Hormones
Survey
Document related concepts
Transcript
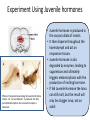
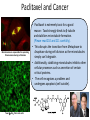
LARGER TERPENES Sesquiterpene - Juvenile hormones Structures of some Juvenile Hormones: • Juvenile Hormones regulate development and reproduction of an insect. • Main role: ensure growth of the larva, while preventing metamorphosis. • Are ultimately produced from IPP and DMAPP. Problem Draw boxes around the isoprene units of MF. Carbon likely added after esterification step Experiment Using Juvenile hormones Effects of mosquito larvae being fed Juvenile Hormone mimics: A. “Larvae Monster” is produced. B. Only partial Metamorphosis into an adult mosquito is observed. • Juvenile hormone is produced in the corpora allata of insects. • It then disperse throughout the haemolymph and act on responsive tissues. • Juvenile hormone is also degraded by enzymes, leading to suppression and ultimately triggers metamorphosis with the production of molting hormone. • If fed Juvenile Hormone the larva can still molt, but the result will only be a bigger larva, not an adult. Sesquiterpene –Juvabione: A Plants Revenge Balsam fir tree Balsam fir tree • In 1965, Sláma and Williams discovered that paper towels made from the wood of the balsam fir released vapors that elicited a potent effect on hemipteran bugs. • They actually mimic juvenile activity to prevent insect reproduction and growth. • These compounds play important roles in conifers against insect induced trauma. • Humans have used this idea to make insecticides. Juvenile Hormone Mimics Methoprene (Most popular): Known Juvenile Hormones (Difficult to Synthesize): O OCH3 O - Used as an insecticide in water cisterns to prevent unwanted mosquito growth. - As used on various crops. Non-toxic to humans. - Unfortunately, it is suspected to be effecting the growth patterns of lobsters through ground water run off. Fenoxycarb: Pyriproxyfen : O O N H O O - Essentially same use as Methoprene, however, it is cheaper to make but is also toxic to fish. N O O O - Used on US cotton crops to deter whitefly growth. Effective against flees on household pets. Left: Fenoxycarb pellets. Right: Household flee pyriproxyfen spray Question: What portions of methoprene and fenoxycarb “map” onto Juvinile hormone? O O OCH3 O O methoprene N H O fenoxycarb O Diterpene (20C’s) – Paclitaxel and Derivatives Cancer cell dividing Pacfic Yew tree Paclitaxel (Taxol) • Earliest account of terpines of this category is reported at 53 B.C., where the Eburone King, Calivolcus (part of the Gallic Empire), committee suicide by drinking Yew bark tea. Not much other history until the 1970’s. • Originally isolated from the bark of the Taxus brevifolia (Pacific Yew), a conifer native North America by Aurthor Barclay who was collecting potential cytoxic natural samples and thought there might be some truth to the “Calivolcus story”. • Is extremely toxic to humans (lethal at 20 mg/kg). • It is also one of the most commonly used chemo-therapeutic agents, and is used for: lung, ovarian, breast cancer, head and neck cancer, and advanced forms of Kaposi's sarcoma. Paclitaxel and Cancer Microtubules are responsible for separating Chromosomes during cell division Taxol-(b-Ala)2-PG in Hela cells • Paclitaxel is extremely toxic for a good reason: Taxol strongly binds to β-tubulin and stabilizes microtubule formation. (Please read 210 and 211 carefully). • This disrupts the transition from Metaphase to Anaphase during cell division as the microtubules simply can’t degrade. • Additionally, stabilizing microtubules inhibits other cellular processes such as secretion of certain critical proteins. • The cell recognizes a problem and undergoes apoptosis (cell suicide). Total Synthesis of Pacitaxel: Nicolaou 1994 Spotlight Reaction: Silyl Ethers as Protection Groups • First introduced in the 1970’s, but highly unsuccessful. The reaction process was markedly improved through chemistry in E. J. Corey’s lab who is now credited with the practical use of silyl ethers for synthesis. • Tert-butyldimethylsilyl group (TBS) was the first heavily utilized group, but now there are a range of silyl groups to match the molecule for ease of adding and removing the group. • These groups are designed to contain different degrees of steric bulk. • For the most part, silyl groups are added to an alcohol through the silyl chloride, and are removed either using an acid or by fluoride. Installation: R + Cl Si R R silyl chloride alcohol OH Base Removal: R O Si R R R O Si R R DMF or CH2Cl2 silyl ether silyl ether HCl, HOAc, or other acid or R + HO Si R R alcohol silanol OH HF or Fluoride (F-) source Example in synthesis: OH O + Cl Si Base: Imidizole TBSO O TBSO OH 2. H3O+ DMF (TBS group) 1. CH3MgBr (protected silyl ether) CH3 HCl OH OH CH3 (TBS is removed) Spotlight Reaction: Silyl Ethers as Protection Groups General Guidelines: • 1o alcohols use: TBDPS or if hindered OH group use TBS • 2o alcohols use: TBS or if hindered OH group use TES • 3o alcohols use: TES or if hindered OH group use TMS Common Protecting Groups: Sterically Hindered Example: Typical Example: OH 1o alcohol OH R R or OH R R R OH o 2 alcohol R R R OH R R R OH 2o alcohol R R R Use in Taxol Synthesis: OH R R or R R OH R R R R OH R R or R R R Pair the correct silyl protection group with the alcohol most appropriate for its use TBDPS TBS TBS TMS TES TBDPS TBS TES TMS Explain why in the Synthesis of Taxol, the TES protection group is more easily removed than the TBS group. Both alcohols silyl protected are 2o and hindered to nearly the same degree. However, TES is less bulky of a silyl protection group and therefore will be remove more readily in the presence of an acid (in this case HF/Pyr). Other Total Syntheses of Pacitaxel Robert A. Holton’s Synthesis: Robert A. Holton Other major syntheses: Danishefsky (1995-Columbia), Wender (1996-Stanford), Mukiayama (1997-Tokyo University), Kuwajima (1998- Tokyo Institute of Technology) Overcoming Issue #1 with Pacitaxel Issue #1: Pacitaxel is terribly insoluble in water and many organic solvents. Why this is an issue: To administer most drugs they must be in a homogenous solution otherwise the drugs effects become less reliable. Solution #1: Find the right combination of solvents to dissolve Pacitaxel. Magic Combination: Ethanol and Cremophor EL (like castor oil). Downside: Tends to result in hypersensitivity in some patients. Solution #2: Find/Produce an analog of Pacitaxel with same activity but better solubility. Result: Taxotere Acetate group is not present Contains tertbutyl group instead of phenyl Taxotere is considerably more soluble in aqueous solutions than is Pacitaxel. As a result, Taxotere is considerably more popular bringing in roughly 3x the annual sales as Pacitaxel. Overcoming Issue #2 with Pacitaxel Issue #2: Without synthesis, Paclitaxel must be obtained from bark from the Pacific yew, the harvesting of which kills the tree in the process. Why this is an issue: It is estimated that annually 7,000 lbs of bark would be needed to supply the world need for Paclitaxel. As this rate the Pacific Yew tree would become extinct in a matter of decades. Solution : The closely related European Yew tree’s pine needles contains a molecule called 10-deacetylbaccatin, which is actually formed in Robert A. Holton’s synthesis of Paclitaxel. Paclitaxel can then be produced from by harvesting 10-deacetylbaccatin and using a synthesis developed by Robert Horton and Iwao Ojima to make Paclitaxel. 10-deacetylbaccatin Pacific Yew Tree bark being harvested European Yew Tree Pacitaxel’s and Taxotere Current Production Steps 3000 kg of European Yew Pine Needles 10-Deacetylbaccatin (1 kg) Steps European Yew Needles can be harvested without killing the tree. As a result, this resource is renewable! Paclitaxel Steps developed by Robert Holton and Iwao Ojima Iwao Ojima Taxotere Robert Holton Other Top Selling Anticancer Drugs Other Top Selling Anticancer Drugs The “Holy Grail” of Cancer Research Mission: Obtain cancer drugs that selective kill cancer cells and not all rapidly dividing cells. Example of one drug: Gleevec which targets Tyrosine Kinases (expressed in much higher levels in cancer cells than in normal dividing cells) Considered the “miracle drug” for chronic myelogenous leukemia. In clinical trials, 53 out of 54 patients recovered after administering the drug for 4 weeks. Clinical trials were cut short for this drug claiming that is was unethical to keep it in trials. Today, it is about 90% effective!