* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A Summary of Organometallic Chemistry
Sol–gel process wikipedia , lookup
Oxidation state wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Metal carbonyl wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Hydroformylation wikipedia , lookup
Spin crossover wikipedia , lookup
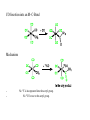
A Summary of Organometallic Chemistry Counting valence electrons (v.e.) with the ionic model 1. Look at the total charge of the complex Ph3P Cl Rh Ph3P PPh3 charge:0 + CO 2– OC Fe OC Co CO charge: -2 charge: +1 2. Look at the charge of the ligands (see table in next page) and calculate the formal oxidation state of the metal and therefore the d electrons at the metal center Ph3P Cl Rh Ph3P PPh3 Rh+ d8 Fe OC + CO 2– OC CO Fe2d10 Co Co3+ d6 3. Add the electrons coming from all ligands Ph3P Cl Rh Ph3P PPh3 1 x 2e- (Cl) 3 x 2e- (PPh3) TOT 8 e- OC Fe OC + CO 2– CO Co 4 x 2e- (CO) 2 x 6e- (Cp) TOT 8 e- TOT 12 e- 4. Add the electrons of both metal and ligands to get the valence electrons (v.e.) Ph3P Cl Rh Ph3P PPh3 16 v.e. CO 2– OC Fe OC + CO 18 v.e. Co 18 v.e. The 18-electron rule The rule helps us to decide whether a complex is likely to be stable. To have a stable transition metal we need to fill all s, p, and d orbitals. These are a total of 9 orbitals, which means 18 electrons. The rule is similar to the 8-electron rule of main group elements. Exceptions to the 18-electrons rule - Early transition metal complexes and/or complexes with bulky ligands are stable with less than 18 v.e. mainly for steric reasons. - Complexes with many π-donor ligands (see below) are stable even with less than 18 v.e. because the πdonation adds electron density to the metal, stabilizing it. - d8 complexes with square planar geometry are stable with 16 v.e. because the dx2-y2 orbital is very high in energy and therefore usually empty. Electron density at the metal center The Pauling’s principle of electroneutrality says that molecules arrange themselves so that their net charge falls between a narrow limit close to neutrality. This means that: 1) Electropositive and high oxidation state elements tend to balance their net charge by binding highly electronegative elements. 2) Elements with intermediate electronegativity prefer to bind to each other Trends in electron density - The higher the oxidation state, the more stable the d orbitals. This means that the high oxidation state metal prefers to bind with ligands donating electron density (see also below). - d-orbitals in 2nd and 3rd row transition metals are more available to be donated. Higher electronegativity and higher electron density is observed going down in the periodic table. - The spectrochemical series of ligands below indicates of how ligands have an active role in modifying the electron density at the metal center. π-donor ligands donate more electron density at the metal than pure σdonor, whereas π-acceptors withdraw electron density from the metal. I– > Br– > Cl– > F– > H2O > NH3 > PMe3 > H, PPh3 > CO π-donors σ-donors π-acceptor donate electron density ————withdraw electron density New Concepts – Oxidative Addition – Insertion / / Elimination – Nucleophilic attack onto a Ligand – H2-Activation Source: Huheey, Crabtree Reductive Elimination Oxidative Addition Reductive Elimination Examples Oxidative Addition: Reductive Elimination: Which Factors determine the tendency of a complex to give oxidative additions? – Tendency to oxidation (electronic effects) → The electron richer the metal, the easier the oxidation: Good donors (e. g., trialkylphosphines) favor oxidative addition. →The tendency to give oxidative addition increases upon descending in a triad: Co(I) < Rh(I) < Ir(I) (mainly because the M–L-bonding energy increases in this order) – Relative stability of the coordination numbers (steric effects) →The tendency to higher coordination numbers within a transition period decreases from left to right. Os(0) > Ir(I) > Pt(II) (all d8 systems): Os(0) has a greater tendency to oxidative addition than Pt(II). →Bulky ligands favor low coordination numbers, and therefore disfavor oxidative addition reactions (if they do not dissociate!) –Strength of the newly formed M–X- and M–Y-bonds relative to X–Y (see CO-insertion below) Mechanism X–Y apolar (H2, O2): Concerted reaction with a three-center transition state: X–Y polar, electrophilic molecule (CH3I, HCl): SN2-Mechanism Insertion (Einschiebung) / Elimination Althought the oxidative addition of X–Y to M gives a complex that formally results from the insertion of a metal atom into the covalent X–Y-bond, the term “insertion” (Einschiebung) is reserved for reactions in which a molecule (which must possess a multiple bond) is inserted into a metal-ligand bond. The oxidation state of the metal remains unchanged: Both reactions have fundamental significance in homogeneous catalysis! Olefin-Insertion into a M–H-Bond: Mechanism: –Ligands involved in an insertion reaction are mutually cis. –The insertion reaction produces a free coordination site. –An elimination from an 18-electron complex can only occur upon dissociation of a ligand. –Also elimination reactions from square planar complexes require a free coordination site: CO-Insertion into an M–C-Bond Mechanism – – No 13C is incorporated into the acetyl group. No 13CO trans to the acetyl group. Mechanism: insertion (Einschiebung) or migration (Wanderung)? Methyl-Migration, CO-Elimination Unambiguously confirmed by the inverse reaction: 25% 25% 50% Predicted for Me-migration observed product distribution 25% 75% Predicted for elimination not observed (Principle of microscopic reversibility: The direct and inverse reaction follow the same elementary steps) Thermodynamics of CO-Insertion Calculated Reaction Enthalpies: J. A. Connor, M. T. Zafarani-Moattar, J. Bickerton, N. I. El Saied, S. Suradi, R. Carson, G. Al Takhin, H. A. Skinner, Organometallics 1982, 1, 1166. Nucleophilic Attack onto a Ligand A nucleophile (Nu–) can attack either the metal (→ substitution) or a coordinated molecule. For instance: The coordination to a metal activates olefins (and related compounds, such as allyls) toward nucleophilic attack (why?). The highest degree of activation occurs in metal complexes that either contain strong π-accepting ligands or are positively charged. Relative Reactivity Nucleophiles of Coordinated Unsaturated Ligands Toward In cationic 18-electron complexes, the reactivity decreases according to: Adapted form: S. G. Davies, M. L. H. Green, D. M. P. Mingos, Tetrahedron 1978, 34, 3047. Note that nucleophilic attack onto an allyl complex is accompanied by 2 e– -reduction of the metal: H2-Activation Some of the catalytic reactions discussed in this course involve the activation of dihydrogen by transition metal complexes. This is a short overview of the possible activation pathways. –Oxidative Addition (catalyst: dihydride complex) –Hydrogenolysis (catalyst:monohydride complex) –Heterolytic H2-Activation (catalyst: monohydride complex) –Homolytic H2-Activation (catalyst: monohydride complex)