* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Compare and Contrast: IBS/IBD
Survey
Document related concepts
Transcript
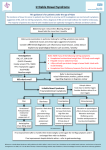
Irritable Bowel Syndrome: Treatment Overview Focusing on Newly Approved Medications Southwest Florida Society of Health System Pharmacists Disclosure • I, or an immediate family member, including spouse or partner, have no financial relationship(s) relevant to the contents of this continuing education activity Jose Barboza, Pharm.D., C.D.E. Faculty Pharmacotherapeutics & Clinical Research University of South Florida College of Pharmacy Objectives Irritable Bowel Syndrome: Definition 1. Describe the pathophysiology of Irritable Bowel Syndrome 2. Review the treatments for Irritable Bowel Syndrome 3. Discuss the use of rifaximin and eluxadoline in treating diarrhea predominant irritable bowel syndrome • Gastrointestinal (GI) syndrome – Abdominal pain or discomfort – Altered bowel habits – Absence of organic cause • Recurring symptoms – Incomplete evacuation – Urgency – Bloating Compare and Contrast: IBS/IBD Irritable Bowel Syndrome (IBS) Pain/ Discomfort Change in Bowel habits Inflammatory Bowel Disease (IBD) Chronic inflammation of the intestines Absence of organic cause Diarrhea (IBS‐D) Crohn’s disease (CD) Constipation (IBS‐C) Ulcerative colitis (UC) – Altered bowel function • Diarrhea predominant (IBS‐D) • Constipation predominant (IBS‐C) • Constipation/ diarrhea (IBS‐M) • Undefined (IBS‐U) Irritable Bowel Syndrome: Diagnosis Rome III Criteria for Irritable Bowel Syndrome Recurrent abdominal pain or discomfort Three episodes per month Last 3 months associated Two or more of… 1 Improvement with defecation 2 Onset associated with change in frequency of stool 3 Onset associated with a change in form of stool Alternating/Mixed (IBS‐M) Am J Gastroenterol, 2009. 104 Suppl 1: p. S1‐35. 1 Irritable Bowel Syndrome: Epidemiology IBS Pathophysiology Disturbed GI motility Visceral hypersensitivity Psychological stress Intestinal microflora and inflammation • Altered levels of 5HT • • • • • Prevalence in US: 10‐15% – More common • Females (~2:1) • Young adulthood – 15% of those affected seek medical attention • Most commonly diagnosed GI condition • 5HT3 and 5HT4 are extensively found in the gut, responsible for secretion, sensitization, and motility – 25‐50% of gastroenterologist referrals Clin Epidemiol. 2014; 6: 71–80. Physiological distribution of 5‐HT • Prucalopride • 5HT4 agonist • IBS-C CNS – 5% • Alosetron • 5HT3 antagonist • IBS-D GI tract – 95% Drugs. 2014 Oct;74(16):1849-70. • Causes altered GI Pathophysiology: • Motility • Sensitivity • Secretions IBS: Dietary Modifications IBS –Positive Diagnosis and Outcome • • • • • • • • Identify concerns Basis for patient’s symptoms Reassurance Cost effective evaluation Involve patient in decision making Provide continuity Set realistic limits No association between negative colonoscopy and improved health related quality of life Gershon, Aliment Pharmacol Ther.,1999 • Avoid caffeine, alcohol, and artificial sweeteners – Can irritate the gut > laxative effect • • • • Evaluate lactose intolerance Rule out Celiac sprue (1%) Rule out gluten intolerance (6%) Low FODMAP diet – Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols – Poorly absorbed by some Gastrointest Endosc 2005; 62:892-9. 2 IBS‐C: Non‐Pharmacologic • Secondary constipation: Correct the cause • Dietary modification: Basis of therapy – Gradually increase fiber – 20‐25 grams/day • Other lifestyle – Exercise – Adjust bowel habits – Increase fluid intake IBS: Fiber Agents and Availability • Agents (Not FDA approved for IBS) – – – – Methylcellulose (Citrucel) Calcium Polycarbophil (FiberCon) Psyllium (Metamucil) Barley malt extract (Maltsupex) • Powders, flakes, granules, tablets, and liquids • Doses vary, typically administered in divided doses IBS: Fiber Precautions • • • • Severely fluid restrictions May cause hypersentitivity Diabetes Fecal impaction or intestinal obstruction – Avoid: intestinal ulcerations, stenosis, and disabling adhesions IBS: Fiber MOA/ Role in Therapy • MOA: Contain hydrophilic polysaccharide derivatives – Absorb water to: Increase bulk, soften the stool, and facilitate peristalsis and elimination – Effects seen in 2‐3 days • Role in therapy: – Safest – Acceptable in pregnancy IBS: Fiber Adverse Effects/Drug Interactions • Adverse effects – Abdominal distention, cramping, and flatulence • Minimized by gradual increase, resolved with continued use • Drug Interactions – Possible binding to digoxin and warfarin – May bind with tetracyclines – Separate other medications by 1‐2 hours IBS: Fiber Evidence • Psyllium/ispaghula husk showed improvement over placebo – NNT=6 (IBS type not differentiated) • Other agents are similar to placebo • Psyllium/ispaghula husk (20‐30 g/day) improves constipation Drugs. 2014 Oct;74(16):1849-70. 3 IBS: Antispasmodics/Anticholinergics • MOA: Relax smooth muscles in the colon and small bowel • Symptomatic relief – Pain • Agents: Peppermint oil, hyoscine, cimetropium, pinaverium, mebeverine, and otilonium • Side effects: Anticholinergic, generally safe IBS: Antidepressants • MOA: Improve dysregulation of neuroenteric pathway • Symptomatic treatment: Abdominal pain – Reserved for patients with severe or refractory pain • Visceral analgesia, changes in motility, smooth muscle relaxation • Agents: – Paroxetine, fluoxetine, citalopram, amitriptyline, and imipramine • Adverse effects (antibiotic dependent): – insomnia, restlessness, sexual dysfunction, nausea, constipation, diarrhea Drugs. 2014 Oct;74(16):1849-70. Drugs. 2014 Oct;74(16):1849-70. IBS: Probiotics • MOA: restore normal flora – Alterations may cause • • • • Diarrhea‐Predominant IBS (IBS‐D) Increased fermentation of food Changes in intestinal motor and sensory function, Mucosal immune activation Malabsorption • Agents: – Lactobacillus, bifidobacterium, streptococcus, others – Limitations in clinical trials • Generally safe IBS‐D: Loperamide • Evaluated in randomized controlled trials for IBS‐D – Effective for treatment of diarrhea – Not FDA approved for IBS • No impact on abdominal bloating or global IBS symptoms • Acute diarrhea: Oral: Initial: 4 mg, followed by 2 mg after each loose stool, up to 16 mg/day IBS‐D: Alosetron MOA/Agent • MOA: Potent and selective 5‐HT3 antagonist – Results in modulation of the enteric nervous system • Alosetron (Lotronex) – Approved in chronic, severe IBS‐D for patients who failed to responded to conventional treatments – Starting dose: 0.5mg BID – Reassess at 4 weeks • No adequate control of symptoms: Increase to 1mg BID – Re‐assess at 4 weeks • No adequate control of symptoms: Discontinue medication ACG Task Force on IBS. Am J Gastro. 2009 4 IBS‐D: Alosetron Restricted Use/Precautions • Adverse effects (dose related) – Constipation – GI discomfort/pain • Restricted FDA use – Females only – Enroll in Prometheus prescribing program • Ischemic colitis (FDA warning) – 1.1 cases/1,000 patient‐years • Precautions: Constipation, ischemic colitis IBS‐D: Alosetron Contraindications • Constipation • intestinal obstruction, stricture, toxic megacolon, gastrointestinal perforation, and/or adhesions • Ischemic colitis, impaired intestinal circulation, thrombophlebitis, or hypercoagulable state • Crohn's disease or ulcerative colitis • Diverticulitis • Severe hepatic impairment • Concomitant fluvoxamine use Chang L et al. Am J Gastro 2010 IBS‐D: Rifaximin • Broad spectrum antibiotic with low bioavailability – <0.4% absorbed • FDA approved • Dose: 550mg TID for 2 weeks – May be repeated up to 2 times – Improvement shown in up to 10 weeks • Showed to improve global IBS symptoms: TARGET 1 & 2 • Adverse effects: Flatulence, abdominal pain, tenesmus, fecal incontinence, nausea, and headaches • High Cost, Xifaxan 550mg (#60)~ $2000 IBS‐D: Eluxadoline (Viberzi) • MOA: kappa and mixed mu and opioid receptor agonist, delta opioid receptor antagonist – Decrease pain and intestine contractility – Shown to improve stool consistency and pain • Global symptoms, QOL, and adequate relief • FDA Approved: IBS3001 and 3002 • Dose: 100mg BID • Unable to tolerate, no gallbladder, mild hepatic impairment: 75mg BID IBS‐D: Eluxadoline • Adverse effects – Constipation (~8% vs. 2.4%), nausea (~7.5% vs. 4.8), vomiting • Contraindications: – Severe hepatic impairment, biliary duct obstruction, diseases of the pancreas, alcoholism 5 IBS‐C: Osmotic Laxatives MOA/ Role in Therapy Constipation‐Predominant IBS (IBS‐C) • MOA: Osmotic agent, causes water retention in the stool and increases stool frequency – Not absorbed systemically – Onset 1‐4 days Role in therapy IBS‐C: Osmotic Laxatives: Dose/ Side Effects/ Contraindications Available with and without electrolytes PEG: Constipation • MOA: Chloride channel activator – Open chloride channels locally on the GI luminal epithelium – Stimulates chloride‐rich fluid secretion into the lumen – Results in softening of the stool and increased motility – Onset: 24‐48 hours 10–30 g or 17–34 g per 120–240 mL QD or BID Not FDA Approved for IBS Side effects Bloating, abdominal discomfort, cramping, flatulence Contraindications GI Obstruction IBS‐C: Lubiprostone Lubiprostone‐ Role in Therapy/ Adverse Effects Agent: Polyethylene Glycol 3350 (Miralax): Rx or OTC IBS‐C: Chloride Channel Activator Lubiprostone‐ MOA/ Agent Low doses for constipation Bowel cleansing before diagnostic or colorectal procedures Safe use chronically, studied in up to 6 months Agent: Lubiprostone (Amitiza) FDA Approved to treat chronic constipation in adults (Rx) IBS‐C: 8 mcg BID with food Constipation: 24mcg BID with food IBS‐C: Guanylate Cyclase‐C agonist Linaclotide‐ MOA/ Agents • MOA: Guanylate cyclase‐C agonist • Role in therapy – Chronic constipation in those who fail first‐line agents • Adverse effects – Nausea (dose dependent), diarrhea, abdominal pain, flatulence, headaches, dyspnea – Pregnancy category C • Contraindication – GI obstruction – Stimulates the secretion of chloride and bicarbonate into the intestinal lumen – Increased intestinal fluid and accelerated GI transit – Decreased visceral pain • Agent: Linaclotide (Linzess) – FDA Approved for IBS‐C: • 290mcg PO QD on an empty stomach • ≥30 minutes prior to the first meal of the day • Chronic Constipation: 145 mcg PO QD on an empty stomach at least 30 minutes prior to the first meal of the day 6 IBS‐C: Serotonin Agonists Prucalopride IBS‐C: Linaclotide Side Effects/ Contraindications • MOA • Side effects: – Diarrhea, abdominal pain, flatulence • Pregnancy category C • Contraindicated – GI obstruction – Children <6 years old • Agents – Prucalopride‐ Not available in USA, available in Europe Treatment Algorithm Diarrhea Predominant Increase: Fiber and Liquid intake Diet: Lactose‐fee and caffeine‐free diet, avoid other diarrhea causing agents Add bulk‐forming laxatives, consider antispasmodic agents Add loperamide or antispasmodic agents Add 5HT‐3 antagonists (alosetron) Add psychotherapeutic behavior modifications, consider antidepressants Drugs. 2014 Oct;74(16):1849-70. Summary No adverse CV effects compared to placebo Altered Bowel Motility: IBS-C Psyllium, osmotic laxatives (PEG), sorbitol/lactulose, lubiprostone, linaclotide, 5HT4 receptor agonists, STW5 Emerging Therapies •IBAT Symptomatic treatment Constipation Predominant Tegaserod‐ withdrawn from the market due to CV adverse events likely due to its actions on hERG channel Prucalopride: no actions on hERG channel and higher affinity to ‐5HT4 Prucalopride has been safely tolerated in clinical trials Altered Bowel Motility: IBS-D Rifaximin, loperamide, 5HT3 receptor antagonists Emerging Therapies: •Bile acid sequestrants •Crofelemer •ASA derivatives Altered Bowel Motility Stress management and patient educations Add 5HT‐4 agonists (prucalopride) or guanylate cyclase‐c agonist (linaclotide) Safety – Selective high affinity 5‐HT4 receptor agonist – Promotes enteric neurons to stimulate the peristaltic reflex, intestinal secretions, and GI motility Pain: Antispasmodics, antidepressants, probiotics, STW5, melatonin Emerging Therapies •Mixed visceral Muopioid •Receptor agonists/antagonists, •Pregabalin •Selective visceral K opioid receptor agonist •H1 receptor antagonists •NK receptor antagonists Pain Bloating Drugs. 2014 Oct;74(16):1849-70. Bloating: Antispasmodics, antiflatulents, probiotics, linaclotide, rifaximin, antidepressants: citalopram, fluxoetine Questions? • IBS and IBD result in similar symptoms and can be difficult to manage – Diagnosis can be difficult – IBS: Increased morbidity – IBD: Increased morbidity and mortality • Cost of therapy can be high • Monitor for medication adverse effects, precautions, and contraindications [email protected] 7