* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Camelford water pollution incident wikipedia , lookup
Wastewater discharge standards in Latin America wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Air well (condenser) wikipedia , lookup
Water pollution wikipedia , lookup
Eutrophication wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
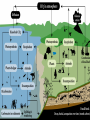
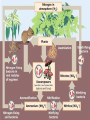
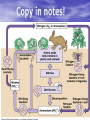
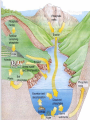
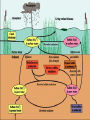
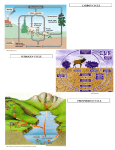
Biogeochemical Cycles • • • • Bio means… – life Geo means… – Of earth: parts of earth are • Land, air, water Chemical means… – Molecules and/or compounds Cycle means… – Repeatedly • Cycling of materials between the environment and organisms • Chemical and biological processes • Examples – Water cycle – Nitrogen cycle – Phosphorus cycle – Carbon cycle Plants obtain nitrogen from nitrogen-fixing bacteria and pass it to other organisms through the food chain Cycles of Matter • No definite beginning or end like food chain • • (remember, energy flow is unidirectional)…matter is recycled Does not use up matter…transforms it Biogeochemical process – Pass same molecule/compound/element through biosphere over and over • Organism to organism • First Part of biosphere (air, land, water) • Second Part of biosphere (air, land, water) Biogeomchemical cycles • Carbon-oxygen • Phosphorus • Water • Nitrogen • Water Cycle • Evaporation: water (in oceans, rivers, lakes) turns to water vapor and • • • • • • rises Transpiration: water evaporates through the stomata of a plant’s leaves and becomes water vapor – Adhesion and cohesion enable water molecules to move from roots to leaves – Stomata: tiny openings in the leaves of plants Condensation: water vapor cools down and condenses in atmosphere to make CLOUDS Precipitation: water returns to surface as rain, snow, ice Run-off: water that moves from mountains and hills to rivers and stream and then eventually to ocean Seepage: water that seeps into the soil and is either taken up by plant roots or becomes part of ground water Ground water: Water that exists beneath the earth's surface in underground streams and aquifers that eventually becomes part of the ocean Water Cycle Water Cycle Impact • Deforestation – Freshwater returns to atmosphere by TRANSPIRATION from tropical forests – Cut down tropical forest=reduce water vapor in air=changes in precipitation patterns and effects ecosystems • Irrigation and household water use – Draws water up from aquifers and rivers – If rate at which H2O is used is FASTER than the water cycle can replace it, rivers and aquifers may run dry (effects ecosystems) Carbon-oxygen cycle • Carbon is the main component of all living things • Carbon is found in glucose, which is the fuel for LIFE! • What other things do we fnd carbon in? Carbon cycle Carbon released as: Carbon is taken in by • Carbon dioxide – Animals and humans release CO2 by cellular respiration – Volcanic eruptions – Burning of fossil fuels (oils) • Methane (CH4) – Grasses and animals release • Bicarbonate ions – Found in rock and released during erosion • Plants • When light is present, plants use photosynthesis to make CO2 and H2O into glucose and oxygen Carbon Cycle Carbon cycle impacts • Atmospheric CO2 levels have steadily risen (more • • industrialized) Burning of wood and fossil fuels release CO2 into atm Deforestation affects carbon cycle – Def: clearing of forests for lumber, agriculture, etc. – Eliminates plants that absorb excess CO2 from the air – “Slash and burn” removes plants and adds CO2 to air • Greenhouse effect – When atmospheric gases trap heat close to Earth’s surface – Makes Earth “liveable”…not a bad thing as long as it is controlled • Global warming (theory) – Theory that there is an overall rise in global temperatures b/c of increase in greenhouse gasses (CO2) – NOT proven Nitrogen cycle • Where is nitrogen found in living things? • Proteins, nucleic acids, and more! • Do you think nitrogen is important? Nitrogen cycleAtmospheric nitrogen (N2) makes up nearly 78% of atmosphere Organisms can not use it in that form. Lightning and bacteria convert nitrogen into usable forms. Terms to know… • Fixation – When N2 gas is made into a useable form (NH4) ammonia • Nitrification – Conversion of ammonia (NH4) into nitrates (NO3-) and nitrites (NO2-) • Assimilation – when plants take up nitrates and nitrites and incorporate into their tissue as amino acids which become proteins • Ammonification/Mineralization – When plants and animals die and decomposers convert amino acids back into ammonia (NH4) and return to soil Nitrogen cycle: Forms of Nitrogen • Nitrogen gas N2 – Nitrogen in atmosphere and nitrogen in decaying organisms needs to be converted – Nitrogen-fixation by bacteria on roots of legumes; Nitrogen GAS Ammonia (sometimes nitrates) • Ammonium (NH4+ )Ammonia NH3 – Nitrification by bacteria in soil; AMMONIA Nitrates and nitrites • Nitrates NO3- and Nitrites NO2- – When oxygen is low in terrestrial and marine environments bacteria convert Nitrogen compounds back into Nitrogen gas N2 • Denitrification when nitrogen containing compounds (Ammonia and nitrates/ites) nitrogen gas by denitrifying bacteria in soil Nitrogen Cycle Nitrogen Cycle Impacts • Humans move large amounts of nitrogen into air or water – Sewage treatments, fertilizers • Lots of Nitrogen in water (and phosphorus) enables algae to grow rapidly on the surface…eutrophication – Definition: excess nutrients in water causes increase in algae (plant) growth which leads to increase in bacterial growth – As algae dies, bacteria that consumes them use up so much available oxygen in the water that there isn't enough for the other marine organisms • Lots of Nitrogen (and sulfur) in Air – Smokestacks and car exhaust pipes release nitrogen dioxide – NO2 reacts with oxygen to make O3 (ozone) in low levels of the atmosphere….this is very bad for living organisms – These nitrogen and sulfur containing compounds mix with water in the air to make NITRIC ACID and SULFURIC ACID – These acids evaporate, condense and come down as ACID PRECIAPTATION (acid rain) – Acid Rain causes damage to soils and aquatic ecosystems Do NOT copy word-for-word!!! -Fertilizers used in farming cause run-off into nearby water=increase in nutrient levels=phytoplankton to grow and reproduce rapidly=algal blooms -This bloom of algae disrupts normal ecosystem & increases bacteria…bacteria use up all the oxygen in the water and there is none left for other marine life…this causes death of many aquatic organisms that need the oxygen -Blooms also block sunlight penetrating the surface and photosynthetic marine plants can’t get sunlight -Blooms also produce toxins that are harmful to higher forms of life -Cause problems along the food chain and affect any animal that feeds on them. Phosphorus cycle • Where do we find phosphorus? • Part of DNA, cell membranes, ATP and ADP, activates and inactivates enzymes • Do you think phosphorus is important? Phosphorus Cycle • NO phosphorus in the atmosphere – Only cycles in soil and land • Found as Phosphorus (P) or Phosphate (PO4-) • Primarily found in the form of mineral apatite – found in rocks and phosphorus minerals Four Phase of Phosphorus Cycle (Terrestrial) • Weathering – Weathering away of phosphate rocks leached phosphate into soil • Plant Uptake – Plants take up P from soil and incorporate into tissues and the animals eat the plants and ASSIMILATE phosphorus into their tissue • Decomposer release – Plants and animals die, decomposer break down tissue and release phosphorus back into soil • Animal exrements – Containphosphorus and return to soil Four Phase of Phosphorus Cycle (Aquatic) • Weathering • • • • – Weathering away of phosphate rocks and soils leach phosphate into rivers and streams Aquatic plant and Phytoplankton Uptake – Take up P in water and incorporate into tissues and the marine vertebrates and invertebrates eat the plants/plankton and ASSIMILATE phosphorus into their tissue Decomposer release – Aquatic plants and animals die, decomposer break down organic phosphate in tissue and release inorganic phosphate back into water Animal excrement – Contain phosphorus and return to water Phosphate Loss – Phosphate lost to marine sediment which is eventually converted into pphosphate0containing rock by geological processes Pollution and the Environment • Pollution: addition of substances to the environment that result in a NEGATIVE effect • Biological Magnification – Animals take in water and nutrients and sometimes pollutants w/them – While energy decreases as it moves up the food chain, toxins increase in potency. – PCBs • Disposed in industrial wastes and Soluble in lipids of dichlor-diphenyltrichlorethylene C14H9Cl5 • animals Concentration of PCBs increases in organisms tissues increase as you move up trophic levels – DDTs • Chemical used to control mosquitoes and crop pests • Soluble in fatty tissue • Birds had high levels of DDT in their tissue and in egg shells, which causes shells to be brittle and young birds cannot survive Damage to Ozone • Ozone: gas in atmosphere (O3) • Ozone absorbs UV radiation from the sun (protects organisms on earth from harmful rays) • Chlorofluorocarbons (CFCs) is a chemical released from aerosol cans, refrigerator units and certain manufacturing processes – Chlorine from CFCs pull off an oxygen from a molecule of O3, making chlorine monoxide, ClO and ozone into regular O2 – ClO binds with another ClO making chlorine peroxide (Cl2 O2) – There’s one less molecule of O3 in the atmosphere to protect organisms from harmful UV radiation – Sun also breaks the chlorine peroxide (Cl2 O2) into chlorine atoms and another O2 molecule and the cycle continues with more carbons interacting with ozone molecules – “Holes in the Ozone” Biodiversity • Definition: # of species in an ecosystem; the variety of ecosystems; the variety of individuals in a species • Why is biodiversity important? – Species in ecosystem are interconnected and depend on each other – If one species disappears, many others affected – Humans depend on biodiversity as well (food, shelter, clothing, medicine) Threats to Biodiversity • Habitat destruction – Pollution, deforestation, desertification, urbanization • Introduced Species • Over Exploitation of resources Conservation Biology • Def: application of biology to counteract the threats to biodiversity – Focus on hot spots • • • • Small geographic areas with high conc. of species Cover less than 1.5% of earth’s surface Hotspots of extinction Contain 1/3 of all plants and vertebrates – Understand Organism’s habitats • Helps maintain org. habitat or create new habitats • Biologists can protect key habitat factors of species – Balance demand for resources • Save species or meet economic and social needs of people • Save a forest to protect and owl but put many loggers out of work? – Planning for a Sustainable future • Ways nations protect environment for future: • Zoned reserves-areas of land that are relatively undisturbed by humans – Encourage long term ecosystem conservations • Buffer zones-areas that surround “zoned” reserve; these buffers are minimally impacted by people...no major envir. disturbances – Ex. Costa Rica- 8 zoned reserves • Sustainable development- developing ways to use natural resources so that the can they are not depleted for future generations – renew themselves and be available to the future… – Ex. Forest corridor between farmlands SUSTAINABILITY!!! • Strategy for using natural resources without depleting them • Using natural resources to provide for human needs without causing long-term environmental harm • Ex. Water restrictions • Ex. Going green