* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A1The eye in detail
Survey
Document related concepts
Idiopathic intracranial hypertension wikipedia , lookup
Mitochondrial optic neuropathies wikipedia , lookup
Blast-related ocular trauma wikipedia , lookup
Visual impairment wikipedia , lookup
Corrective lens wikipedia , lookup
Dry eye syndrome wikipedia , lookup
Photoreceptor cell wikipedia , lookup
Contact lens wikipedia , lookup
Retinitis pigmentosa wikipedia , lookup
Keratoconus wikipedia , lookup
Diabetic retinopathy wikipedia , lookup
Vision therapy wikipedia , lookup
Visual impairment due to intracranial pressure wikipedia , lookup
Transcript
A. The Eye
A1. Eye in detail
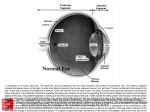
EYE ANATOMY
A guide to the many parts of the human eye and how they function.
The ability to see is dependent on the actions of several structures in and around the eyeball.
The graphic below lists many of the essential components of the eye's optical system.
When you look at an object, light rays are reflected from the object to the cornea, which is
where the miracle begins. The light rays are bent, refracted and focused by the cornea, lens,
and vitreous. The lens' job is to make sure the rays come to a sharp focus on the retina. The
resulting image on the retina is upside-down. Here at the retina, the light rays are converted
to electrical impulses which are then transmitted through the optic nerve, to the brain, where
the image is translated and perceived in an upright position!
Think of the eye as a camera. A camera needs a lens and a film to produce an image. In the
same way, the eyeball needs a lens (cornea, crystalline lens, vitreous) to refract, or focus the
light and a film (retina) on which to focus the rays. If any one or more of these components is
not functioning correctly, the result is a poor picture. The retina represents the film in our
camera. It captures the image and sends it to the brain to be developed. The macula is the
highly sensitive area of the retina. The macula is responsible for our critical focusing vision.
It is the part of the retina most used. We use our macula to read or to stare intently at an
object.
http://www.stlukeseye.com/Anatomy.asp
Angle Structures
The area in the anterior chamber where the cornea and iris join is known as the angle. This is
comprised of several structures that make up the eye's drainage system. The angle structures
include: the outermost part of the iris, the front of the ciliary body, the trabecular meshwork,
and the Canal of Schlemm.
Aqueous is formed in the ciliary body behind the iris. It flows through the pupillary space
into the anterior chamber. From there, the fluid travels into the angle structures and drains
from the eye.
As the aqueous fluid leaves the angle, it passes through a filter called the
trabecular meshwork. After leaving the trabecular meshwork, the
aqueous travels through a tiny channel in the sclera called the Canal of
Schlemm. The aqueous flows into other tiny channels and eventually into
the eye's blood vessels.
The production and drainage of aqueous fluid determines the eye's
intraocular pressure (IOP).
Aqueous Humor
The aqueous is the thin, watery fluid that fills the space between the cornea and the iris
(anterior chamber). It is continually produced by the ciliary body, the part of the eye that lies
just behind the iris. This fluid nourishes the cornea and the lens and gives the front of the eye
its form and shape.
Choroid
The choroid lies between the retina and sclera. It is composed of layers of blood vessels that
nourish the back of the eye. The choroid connects with the ciliary body toward the front of
the eye and is attached to edges of the optic nerve at the back of the eye.
Ciliary Body
The ciliary body lies just behind the iris. Attached to the ciliary body are tiny
fiber "guy wires" called zonules. The crystalline lens is suspended inside the
eye by the zonular fibers. Nourishment for the ciliary body comes from blood
vessels which also supply the iris.
One function of the ciliary body is the production of aqueous humor, the clear
fluid that fills the front of the eye. It also controls accommodation by
changing the shape of the crystalline lens. When the ciliary body contracts,
the zonules relax. This allows the lens to thicken, increasing the eye's ability
to focus up close. When looking at a distant object, the ciliary body relaxes, causing the
zonules to contract. The lens becomes thinner, adjusting the eye's focus for distance vision.
With age, everyone develops a condition known as presbyopia. This occurs as the ciliary
body muscle and lens gradually lose elasticity, causing difficulty reading.
Conjunctiva
The conjunctiva is the thin, transparent tissue that covers the outer surface of the eye. It
begins at the outer edge of the cornea, covering the visible part of the sclera, and lining the
inside of the eyelids. It is nourished by tiny blood vessels that are nearly invisible to the
naked eye.
The conjunctiva also secretes oils and mucous that moisten and lubricate the eye.
Cornea
The cornea is the transparent, dome-shaped window covering the front of the eye. It is a
powerful refracting surface, providing 2/3 of the eye's focusing power. Like the crystal on a
watch, it gives us a clear window to look through.
Because there are no blood vessels in the cornea, it is normally clear and has a shiny surface.
The cornea is extremely sensitive - there are more nerve endings in the cornea than anywhere
else in the body.
The adult cornea is only about 1/2 millimeter thick and is comprised of 5 layers: epithelium,
Bowman's membrane, stroma, Descemet's membrane and the endothelium.
The layers of the cornea
The epithelium is layer of cells that cover the surface of the cornea. It is only about 5-6 cell
layers thick and quickly regenerates when the cornea is injured. If the injury penetrates more
deeply into the cornea, it may leave a scar. Scars leave opaque areas, causing the corneal to
lose its clarity and luster.
Boman's membrane lies just beneath the epithelium. Because this layer is very tough and
difficult to penetrate, it protects the cornea from injury.
The stroma is the thickest layer and lies just beneath Bowman's. It is composed of tiny
collagen fibrils that run parallel to each other. This special formation of the collagen fibrils
gives the cornea its clarity.
Descemet's membrane lies between the stroma and the endothelium. The endothelium is just
underneath Descemet's and is only one cell layer thick. This layer pumps water from the
cornea, keeping it clear. If damaged or disease, these cells will not regenerate.
Tiny vessels at the outermost edge of the cornea provide nourishment, along with the aqueous
and tear film.
Extraocular Muscles
The six tiny muscles that surround the eye and control its movements are known as the
extraocular muscles (EOMs). The primary function of the four rectus muscles is to control
the eye's movements from left to right and up and down. The two oblique muscles move the
eye rotate the eyes inward and outward.
All six muscles work in unison to move the eye. As one contracts, the opposing muscle
relaxes, creating smooth movements. In addition to the muscles of one eye working together
in a coordinated effort, the muscles of both eyes work in unison so that the eyes are always
aligned.
Eyelids
The eyelids protect the eyes from the environment, injury and light. They also maintain a
smooth corneal surface by spreading tears evenly over the eye. The lids are composed of an
outer layer of skin, a middle layer of muscle and tissue that gives them form, and an inner
layer of moist conjunctival tissue.
Several muscles work together to control the actions of the lids. Located in the middle layer
of the eyelid is the orbicularis oculi, a circular muscle that closes the lids. The levator muscle
is attached inside the upper lid and elevates it. A smooth muscle called Mueller's gives the
lids tone and helps maintain elasticity.
Tiny oil-producing meibomian glands line the inner edge of the lids. These glands produce
oil that lubricates the eye. Rows of lashes protect the eyes from the elements and debris.
Not only do the eyelids provide protection and moisture, they display expression and
emotions that are an important part of our individuality.
Iris
The colored part of the eye is called the iris. It controls light levels inside the eye similar to
the aperture on a camera. The round opening in the center of the iris is called the pupil. The
iris is embedded with tiny muscles that dilate (widen) and constrict (narrow) the pupil size.
The sphincter muscle lies around the very edge of the pupil. In bright light, the sphincter
contracts, causing the pupil to constrict. The dilator muscle runs radially through the iris, like
spokes on a wheel. This muscle dilates the eye in dim lighting.
The iris is flat and divides the front of the eye (anterior chamber) from the back of the eye
(posterior chamber). Its color comes from microscopic pigment cells called melanin. The
color, texture, and patterns of each person's iris are as unique as a fingerprint.
Lens
The crystalline lens is located just behind the iris. Its purpose is to focus light onto the retina.
The nucleus, the innermost part of the lens, is surrounded by softer material called the cortex.
The lens is encased in a capsular-like bag and suspended within the eye by tiny "guy wires"
called zonules.
In young people, the lens changes shape to adjust for close or
distance vision. This is called accommodation. With age, the lens
gradually hardens, diminishing the ability to accommodate.
Macula
he macula is located roughly in the center of the retina, temporal to the
optic nerve. It is a small and highly sensitive part of the retina responsible
for detailed central vision. The fovea is the very center of the macula. The
macula allows us to appreciate detail and perform tasks that require central
vision such reading.
Optic Nerve
The optic nerve transmits electrical impulses from the retina to the brain. It connects to the
back of the eye near the macula. When examining the back of the eye, a portion of the optic
nerve called the optic disc can be seen.
The retina's sensory receptor cells of retina are absent from the optic nerve. Because of this,
everyone has a normal blind spot. This is not normally noticeable because the vision of both
eyes overlaps.
Pupil
The pupil is the opening in the center of the iris. The size of the pupil determines the amount
of light that enters the eye. The pupil size is controlled by the dilator and sphincter muscles of
the iris. Doctors often evaluate the reaction of pupils to light to determine a person's
neurological function.
Retina
The retina is a multi-layered sensory tissue that lines the back of the eye. It contains millions
of photoreceptors that capture light rays and convert them into electrical impulses. These
impulses travel along the optic nerve to the brain where they are turned into images.
There are two types of photoreceptors in the retina: rods and cones. The retina contains
approximately 6 million cones. The cones are contained in the macula, the portion of the
retina responsible for central vision. They are most densely
packed within the fovea, the very center portion of the macula.
Cones function best in bright light and allow us to appreciate
color.
There are approximately 125 million rods. They are spread
throughout the peripheral retina and function best in dim
lighting. The rods are responsible for peripheral and night
vision.
This photograph shows a normal retina with blood vessels that branch from the optic nerve,
cascading toward the macula.
Sclera
The sclera is commonly known as "the white of the eye." It is the tough, opaque tissue that
serves as the eye's protective outer coat. Six tiny muscles connect to it around the eye and
control the eye's movements. The optic nerve is attached to the sclera at the very back of the
eye.
In children, the sclera is thinner and more translucent, allowing the underlying tissue to show
through and giving it a bluish cast. As we age, the sclera tends to become more yellow.
Tear Film
Tears are formed by tiny glands that surround the eye. The tear film is comprised of three
layers: oil, water, and mucous. The lower mucous layer serves as an anchor for the tear film
and helps it adhere to the eye. The middle layer is comprised of water. The upper oil layer
seals the tear film and prevents evaporation.
The tear film serves several purposes: it keeps the eye moist, creates a smooth surface for
light to pass through the eye, nourishes the front of the eye, and provides protection from
injury and infection.
Tear Production System
The eye's tears are composed of three layers: oil, water and mucous. The outermost oily
layer is produced by the meibomian glands which line the edge of the eyelids. The watery
portion of the tear film is produced by the lacrimal gland. This gland lies underneath the
outer orbital rim bone, just below the eyebrow. The mucous layer comes from microscopic
goblet cells in the conjunctiva.
With each blink, the eyelids sweep across the eye, spreading the tear film evenly across the
surface. The blinking motion of the eyelids forces the tears into tiny drains found at the inner
corners of the upper and lower eyelids. These drains are called puncta (plural for punctum).
The tear film travels from the puncta into the upper and lower canaliculus, which empty into
the lacrimal sac. The lacrimal sac drains into the nasolacrimal duct which connects to the
nasal passage. This connection between the tear production system and the nose is the reason
your nose runs when you cry. Some patients can actually taste eye drops
as they drain from the nasal passage into the throat
Vitreous
The vitreous is a thick, transparent substance that fills the center of the eye. It is composed
mainly of water and comprises about 2/3 of the eye's volume, giving it form and shape. The
viscous properties of the vitreous allow the eye to return to its normal shape if compressed.
In children, the vitreous has a consistency similar to an egg white. With age it gradually thins
and becomes more liquid. The vitreous is firmly attached to certain areas of the retina. As the
vitreous thins, it separates from the retina, often causing floaters.
http://www.stlukeseye.com
RETINA
The retina is the light sensitive inner layer of the eye, which receives images formed by the
lens and transmits them through the optic nerve to the brain. It is comparable to the film in a
camera. In vertebrate embryonic development, the retina and the optic nerve originate as
outgrowths of the developing brain. Hence, the retina is part of the central nervous system
(CNS). It is the only part of the CNS that can be imaged directly.
The vertebrate retina contains photoreceptor cells (rods and cones) that respond to light; the
resulting neural signals then undergo complex processing by other neurons of the retina. The
retinal output takes the form of action potentials in retinal ganglion cells whose axons form
the optic nerve. Several important features of visual perception can be traced to the retinal
encoding and processing of light.
A third category of photosensitive cells in the retina is not involved in vision. A small
proportion of the ganglion cells, about 2% in humans, contain the pigment melanopsin and
respond primarily to blue light, about 470 nm. The signals from these cells do not go through
the optic nerve, and thus can function in many totally blind individuals. The information
about light goes through the retinohypothalamic tract directly to the SCN (suprachiasmatic
nucleus) and are necessary for the organism's adjustment of its circadian rhythms.
The unique structure of the blood vessels in the retina has been used for biometric
identification.
Anatomy of vertebrate retina
Section of retina.
The vertebrate retina has ten distinct layers. From innermost to outermost, they include:
1. Inner limiting membrane - Müller cell footplates
2. Nerve fiber layer
3. Ganglion cell layer - Layer that contains nuclei of ganglion cells and gives rise to
optic nerve fibers.
4. Inner plexiform layer
5. Inner nuclear layer
6. Outer plexiform layer - In the macular region, this is known as the Fiber layer of
Henle.
7. Outer nuclear layer
8. External limiting membrane - Layer that separates the inner segment portions of the
photoreceptors from their cell nuclei.
9. Photoreceptor layer - Rods / Cones
10. Retinal pigment epithelium
Physical structure of human retina
In adult humans the entire retina is 72% of a sphere about 22 mm in diameter. An area of the
retina is the optic disc, sometimes known as "the blind spot" because it lacks photoreceptors.
It appears as an oval white area of 3 mm². Temporal (in the direction of the temples) to this
disc is the macula. At its center is the fovea, a pit that is most sensitive to light and is
responsible for our sharp central vision. Human and non-human primates possess one fovea as
opposed to certain bird species such as hawks who actually are bifoviate and dogs and cats
who possess no fovea but a central band known as the visual streak. Around the fovea extends
the central retina for about 6 mm and then the peripheral retina. The edge of the retina is
defined by the ora serrata. The length from one ora to the other (or macula), the most sensitive
area along the horizontal meridian is about 3.2 mm.
Retina's simplified axial organization. The retina is a stack of several neuronal layers. Light is
concentrated from the eye and passes across these layers (from left to right) to hit the
photoreceptors (right layer). This elicits chemical transformation mediating a propagation of
signal to the bipolar and horizontal cells (middle yellow layer). The signal is then propagated
to the amacrine and ganglion cells. These neurons ultimately may produce action potentials
on their axons. This spatiotemporal pattern of spikes determines the raw input from the eyes
to the brain.
In section the retina is no more than 0.5 mm thick. It has three layers of nerve cells and two of
synapses. The optic nerve carries the ganglion cell axons to the brain and the blood vessels
that open into the retina. As a byproduct of evolution, the ganglion cells lie innermost in the
retina while the photoreceptive cells lie outermost. Because of this arrangement, light must
first pass through the thickness of the retina before reaching the rods and cones. However it
does not pass through the epithelium or the choroid (both of which are opaque).
The white blood cells in the capillaries in front of the photoreceptors can be perceived as tiny
bright moving dots when looking into blue light. This is known as the blue field entoptic
phenomenon (or Scheerer's phenomenon).
Between the ganglion cell layer and the rods and cones there are two layers of neuropils
where synaptic contacts are made. The neuropil layers are the outer plexiform layer and the
inner plexiform layer. In the outer the rod and cones connect to the vertically running bipolar
cells and the horizontally oriented horizontal cells connect to ganglion cells.
The central retina is cone-dominated and the peripheral retina is rod-dominated. In total there
are about seven million cones and a hundred million rods. At the centre of the macula is the
foveal pit where the cones are smallest and in a hexagonal mosaic, the most efficient and
highest density. Below the pit the other retina layers are displaced, before building up along
the foveal slope until the rim of the fovea or parafovea which is the thickest portion of the
retina. The macula has a yellow pigmentation from screening pigments and is known as the
macula lutea.
Vertebrate and cephalopod retina differences
The vertebrate retina is inverted in the sense that the light sensing cells sit at the back side of
the retina, so that light has to pass through a layer of neurons before it reaches the
photoreceptors. By contrast, the cephalopod retina is everted: the photoreceptors are located at
the front side of the retina, with processing neurons behind them. Because of this,
cephalopods do not have a blind spot.
The cephalopod retina does not originate as an outgrowth of the brain, as the vertebrate one
does. This shows that vertebrate and cephalopod eyes are not homologous but have evolved
separately.
Physiology
An image is produced by the "patterned excitation" of the retinal receptors, the cones and
rods. The excitation is processed by the neuronal system and various parts of the brain
working in parallel to form a representation of the external environment in the brain.
The cones respond to bright light and mediate high-resolution vision and colour vision. The
rods respond to dim light and mediate lower-resolution, black-and-white, night vision. It is a
lack of cones sensitive to red, blue, or green light that causes individuals to have deficiencies
in colour vision or various kinds of colour blindness. Humans and old world monkeys have
three different types of cones (trichromatic vision) while other mammals lack cones with red
sensitive pigment and therefore have poorer (dichromatic) colour vision.
When light falls on a receptor it sends a proportional response synaptically to bipolar cells
which in turn signal the retinal ganglion cells. The receptors are also 'cross-linked' by
horizontal cells and amacrine cells, which modify the synaptic signal before the ganglion
cells. Rod and cone signals are intermixed and combine, although rods are mostly active in
very poorly lit conditions and saturate in broad daylight, while cones function in brighter
lighting because they are not sensitive enough to work at very low light levels.
Despite the fact that all are nerve cells, only the retinal ganglion cells and few amacrine cells
create action potentials. In the photoreceptors, exposure to light hyperpolarizes the membrane
in a series of graded shifts. The outer cell segment contains a photopigment. Inside the cell the
normal levels of cGMP keeps the Na+ channel open and thus in the resting state the cell is
depolarised. The photon causes the retinal bound to the receptor protein to isomerise to transretinal. This causes receptor to activate multiple G-proteins. This in turn causes the Gasubunit of the protein to bind and degrade cGMP inside the cell which then cannot bind to the
CNG Na+ channels. Thus the cell is hyperpolarised. The amount of neurotransmitter released
is reduced in bright light and increases as light levels fall. The actual photopigment is
bleached away in bright light and only replaced as a chemical process, so in a transition from
bright light to darkness the eye can take up to thirty minutes to reach full sensitivity (see dark
adaptation).
In the retinal ganglion cells there are two types of response, depending on the receptive field
of the cell. The receptive fields of retinal ganglion cells comprise a central approximately
circular area, where light has one effect on the firing of the cell, and an annular surround,
where light has the opposite effect on the firing of the cell. In ON cells, an increment in light
intensity in the centre of the receptive field causes the firing rate to increase. In OFF cells, it
makes it decrease. In a linear model, this response profile is well described by a Difference of
Gaussians and is the basis for edge detection algorithms. Beyond this simple difference
ganglion cells are also differentiated by chromatic sensitivity and the type of spatial
summation. Cells showing linear spatial summation are termed X cells (also called
"parvocellular", "P", or "midget" ganglion cells), and those showing non-linear summation are
Y cells (also called "magnocellular, "M", or "parasol" retinal ganglion cells), although the
correspondence between X and Y cells (in the cat retina) and P and M cells (in the primate
retina) is not as simple as it once seemed.
In the transfer of signal to the brain, the visual pathway, the retina is vertically divided in two,
a temporal half and a nasal half. The axons from the nasal half cross the brain at the optic
chiasma to join with axons from the temporal half of the other eye before passing into the
lateral geniculate body.
Although there are more than 130 million retinal receptors, there are only approximately 1.2
million fibres (axons) in the optic nerve so a large amount of pre-processing is performed
within the retina. The fovea produces the most accurate information. Despite occupying about
0.01% of the visual field (less than 2° of visual angle), about 10% of axons in the optic nerve
are devoted to the fovea. The resolution limit of the fovea has been determined at around
10,000 points. The information capacity is estimated at 500,000 bits per second (for more
information on bits, see information theory) without colour or around 600,000 bits per second
including colour.
Spatial Encoding
On-centers and off-centers of the retina
The retina, unlike a camera, does not simply relay a picture to the brain. The retina spatially
encodes (compresses) the image to fit the limited capacity of the optic nerve. Compression is
necessary because there are 100 times more Photoreceptor cells than ganglion cells as
mentioned above. The retina does so by "decorrelating" the incoming images in a manner to
be described below. These operations are carried out by the center surround structures as
implemented by the bipolar and ganglion cells.
There are two types of center surround structures in the retina -- on-centers and off-centers.
On-centers have a positively weighted center and a negatively weighted surround. Off-centers
are just the opposite. Positive weighting is more commonly known as excitatory and negative
weighting is more commonly known as inhibitory.
These center surround structures are not physical in the sense that you cannot see them by
staining samples of tissue and examining the retina's anatomy. The center surround structures
are logical (i.e., mathematically abstract) in the sense that they depend on the connection
strengths between ganglion and bipolar cells. It is believed that the connection strengths
between cells is caused by the number and types of ion channels embedded in the synapses
between the ganglion and bipolar cells. Stephen Kuffler in the 1950s was the first person to
begin to understand these center surround structures in the retina of cats. See Receptive field
for figures and more information on center surround structures. See chapter 3 of David
Hubel's on-line book (listed below) for an excellent introduction.
The center surround structures are mathematically equivalent to the edge detection algorithms
used by computer programmers to extract or enhance the edges in a digital photograph. Thus
the retina performs operations on the image to enhance the edges of objects within its visual
field. For example, in a picture of a dog, a cat and a car, it is the edges of these objects that
contain the most information. In order for higher functions in the brain (or in a computer for
that matter) to extract and classify objects such as a dog and a cat, the retina is the first step to
separating out the various objects within the scene.
As an example, the following matrix is at the heart of the computer algorithm that implements
edge detection. This matrix is the computer equivalent to the center surround structure. In this
example, each box (element) within this matrix would be connected to one photoreceptor. The
photoreceptor in the center is the current receptor being processed. The center photoreceptor
is multiplied by the +1 weight factor. The surrounding photoreceptors are the "nearest
neighbors" to the center and are multiplied by the -1/8 value. The sum of all nine of these
elements is finally calculated. This summation is repeated for every photoreceptor in the
image by shifting left to the end of a row and then down to the next line.
The total sum of this matrix is zero if all the inputs from the nine photoreceptors are the same
value. The zero result indicates the image was uniform (non-changing) within this small
patch. Negative or positive sums mean something was varying (changing) within this small
patch of nine photoreceptors.
-1/8 -1/8 -1/8
-1/8 +1 -1/8
-1/8 -1/8 -1/8
The above matrix is only an approximation to what really happens inside the retina. First, the
table is square while the center surround structures in the retina are circular. Second, neurons
operate on spike trains traveling down nerve cell axons. Computers operate on a single
constant number from each input pixel (the computer equivalent of a photoreceptor). Third,
the retina performs all these calculations in parallel while the computer operates on each pixel
one at a time. There are no repeated summations and shifting as there would be in a computer.
Forth, the horizontal and amacrine cells play a significant role in this process but that is not
represented here.
Here is an example of an input image and how edge detection would modify it.
Once the image is spatially encoded by the center surround structures, the signal is sent out
the optical nerve (via the axons of the ganglion cells) through the optic chiasm to the LGN
(lateral geniculate nucleus). The exact function of the LGN is unknown at this time. The
output of the LGN is then sent to the back of the brain. Specifically the output of the LGN
"radiates" out to the V1 Primary visual cortex.
Simplified Signal Flow: Photoreceptors ==> Bipolor ==> Ganglion ==> Chiasm ==> LGN
==> V1 cortex
Diseases and disorders
There are many inherited and acquired diseases or disorders that may affect the retina. Some
of them include:
•
•
•
•
•
•
Retinitis pigmentosa is a group of genetic diseases that affect the retina and causes the
loss of night vision and peripheral vision.
Macular degeneration describes a group of diseases characterized by loss of central
vision because of death or impairment of the cells in the macula.
Cone-rod dystrophy (CORD) describes a number of diseases where vision loss is
caused by deterioration of the cones and/or rods in the retina.
In retinal separation, the retina detaches from the back of the eyeball. Ignipuncture is
an outdated treatment method.
Both hypertension and diabetes mellitus can cause damage to the tiny blood vessels
that supply the retina, leading to hypertensive retinopathy and diabetic retinopathy.
Retinoblastoma is a cancer of the retina.
•
Retinal diseases in dogs include retinal dysplasia, progressive retinal atrophy, and
sudden acquired retinal degeneration.
Diagnosis and treatment
A number of different instruments are available for the diagnosis of diseases and disorders
affecting the retina. An ophthalmoscope is used to examine the retina. Recently, adaptive
optics has been used to image individual rods and cones in the living human retina.
The electroretinogram is used to measure non-invasively the retina's electrical activity, which
is affected by certain diseases. A relatively new technology, now becoming widely available,
is optical coherence tomography (OCT). This non-invasive technique allows one to obtain a
3D volumetric or high resolution cross-sectional tomogram of the retinal fine structure with
histologic-quality.
http://en.wikipedia.org/wiki/Retina
VISUAL IMPAIRMENT
Visual impairment or vision impairment is vision loss that constitutes a significant
limitation of visual capability resulting from disease, trauma, or a congenital or degenerative
condition that cannot be corrected by conventional means, including refractive correction,
medication, or surgery. This functional loss of vision is typically defined to manifest with 1)
best corrected visual acuity of less than 20/60, or significant central field defect, 2) significant
peripheral field defect including homonymous or heteronymous bilateral visual field defect or
generalized contraction or constriction of field, or 3) reduced peak contrast sensitivity either
of the above conditions.
1. Partially sighted indicates some type of visual problem, with a need of person to
receive special education in some cases;
2. Low vision generally refers to a severe visual impairment, not necessarily limited to
distance vision. Low vision applies to all individuals with sight who are unable to read
the newspaper at a normal viewing distance, even with the aid of eyeglasses or contact
lenses. They use a combination of vision and other senses to learn, although they may
require adaptations in lighting or the size of print, and, sometimes, braille;
1. Myopic - unable to see distant objects clearly, commonly called near-sighted or
short-sighted
2. Hyperopic - unable to see close objects clearly, commonly called far-sighted or
long-sighted
3. Legally blind indicates that a person has less than 20/200 vision in the better eye or a
very limited field of vision (20 degrees at its widest point); and
4. Totally blind students learn via braille or other non-visual media.
Visual impairment is the consequence of a functional loss of vision, rather than the eye
disorder itself. Eye disorders which can lead to visual impairments can include retinal
degeneration, albinism, cataracts, glaucoma, muscular problems that result in visual
disturbances, corneal disorders, diabetic retinopathy, congenital disorders, and infection."
Visual impairment can also be caused by brain and nerve disorders, in which case it is usually
termed cortical visual impairment (CVI).
The American Medical Association's Guides to the Evaluation of Permanent Impairment
attempts to provide "a standardized, objective approach to evaluating medical impairments."
The Visual System chapter "provides criteria for evaluating permanent impairment of the
visual system as it affects an individual's ability to perform activities of daily living." The
Guide has estimated that the loss of one eye equals 25% impairment of the visual system and
24% impairment of the whole person; total loss of vision in both eyes is considered to be
100% visual impairment and 85% impairment of the whole person.
Visual impairments have considerable economic impact on even developed countries.
http://en.wikipedia.org/wiki/Visual_impairment
FIELD OF VISION
Field of vision is the angular extent of the observable world that is seen at any given moment.
Different animals have different fields of view, depending on the placement of the eyes.
Humans have an almost 180-degree forward-facing field of view, while some birds have a
complete or nearly-complete 360-degree field of view. In addition the vertical range of the
field of view may vary.
The range of visual abilities is not uniform across a field of view, and varies from animal to
animal. For example, binocular vision, which is important for depth perception, only covers
140 degrees of the field of vision in humans; the remaining peripheral 40 degrees have no
binocular vision (because of the lack of overlap in the images from either eye for those parts
of the field of view). The aforementioned birds would have a scant 10 or 20 degrees of
binocular vision.
Similarly, color vision and the ability to perceive shape and motion vary across the field of
view; in humans the former is concentrated in the center of the visual field, while the latter
tends to be much stronger in the periphery. This is due to the much higher concentration of
color-sensitive cone cells in the fovea, the central region of the retina, in comparison to the
higher concentration of motion-sensitive rod cells in the periphery. Since cone cells require
considerably brighter light sources to be activated, the result of this distribution is that
peripheral vision is much stronger at night relative to binocular vision.
Conversions
Many optical instruments, particularly binoculars or spotting scopes, are advertised with their
field of view specified in one of two ways: angular field of view, and linear field of view.
Angular field of view is typically specified in degrees, while linear field of view is a ratio of
lengths. For example, binoculars with a 5.8 degree (angular) field of view might be advertised
as having a (linear) field of view of 305 feet per 1000 yards or 102 mm per meter. As long as
the FOV is less than about 10 degrees or so, the following approximation formulas allow one
to convert between linear and angular field of view. Let A be the angular field of view in
degrees. Let L be the linear field of view in feet per 1000 yards. Let M be the linear field of
view in millimeters per meter. Then:
http://en.wikipedia.org/wiki/Field_of_view
LOW VISION
Low vision is a subspecialty within the professions of optometry and ophthalmology and
opticianry dealing with individuals who have less than normal vision even with the most
accurate conventional prescription available. It can be a result of either congenital or acquired
factors. An example of the former is Leber's congenital amaurosis and of the latter age-related
macular degeneration.
Classifying Low Vision
Anyone with noncorrectable reduced vision is considered to be visually impaired, and can
have a wide range of causes. The World Health Organization uses the following
classifications of visual impairment. When the vision in the better eye with best possible
glasses correction is:
•
•
•
•
•
•
20/30 to 20/60 : is considered mild vision loss, or near-normal vision
20/70 to 20/160 : is considered moderate visual impairment, or moderate low vision
20/200 to 20/400 : is considered severe visual impairment, or severe low vision
20/500 to 20/1,000 : is considered profound visual impairment, or profound low vision
less than 20/1,000 : is considered near-total visual impairment, or near total blindness
No Light Perception : is considered total visual impairment, or total blindness
There are also levels of visual impairment based on visual field loss (loss of peripheral
vision).
In the United States, any person with vision that cannot be corrected to better than 20/200 in
the best eye, or who has 20 degrees (diameter) or less of visual field remaining, is considered
to be "legally blind" or eligible for disability classification and possible inclusion in certain
government sponsored programs.
Magnitude of visual impairment
•
Globally, in 2002 more than 161 million people were visually impaired, of whom 124
million people had low vision
and 37 million were blind. However, refractive error as a cause of visual impairment was not
included, which implies that the actual global magnitude of visual impairment is greater.
•
Worldwide for each blind person, an average of 3.4 people have low vision, with
country and regional variation ranging from 2.4 to 5.5.
Pathologies which may cause vision acuity loss
•
•
•
•
•
•
•
•
•
•
•
Cataracts
Glaucoma
Uveitis
Macular degeneration
Corneal opacity
Trachoma
Diabetic retinopathy
Myopia magna
Stargardt's disease
Albinism
Retinitis pigmentosa
Since the estimates of the 1990s, new data based on the 2002 global population show a
reduction in the number of people who are blind or visually impaired, and those who are blind
from the effects of infectious diseases, but an increase in the number of people who are blind
from conditions related to longer life spans. This new information underscores the need to
modify the health care agenda to include the management of the diseases that are now
becoming prevalent.
Distribution of visual impairment
By age: Visual impairment is unequally distributed across age groups. More than 82% of all
people who are blind are 50 years of age and older, although they represent only 19% of the
world's population. Due to the expected number of years lived in blindness (blind years),
childhood blindness remains a significant problem, with an estimated 1.4 million blind
children below age 15.
By gender: Available studies consistently indicate that in every region of the world, and at all
ages, females have a significantly higher risk of being visually impaired than males.
Geographically: Visual impairment is not distributed uniformly throughout the world. More
than 90% of the world's visually impaired live in developing countries.
Low Vision, its lifestyle implications and rehabilitation
Visual impairments may take many forms and be of varying degrees. Visual acuity alone is
not always a good predictor of the degree of problems a person may have. Someone with
relatively good acuity (e.g., 20/40) can have difficulty with daily functioning, while someone
with worse acuity (e.g., 20/200) may function reasonably well if their visual demands are not
great.
Some people who fall into this category can use their considerable residual vision - their
remaining sight - to complete daily tasks without relying on alternative methods. The role of a
low vision specialist (optometrist or ophthalmologist) is to maximize the functional level of a
patient's vision by optical or non-optical means. Primarily, this is by use of magnification in
the form of telescopic systems for distance vision and optical or electronic magnification for
near tasks.
People with significantly reduced acuity may benefit from training conducted by individuals
trained in the provision of technical aids. Rehabilitation professionals, some of whom are
connected to an agency for the blind, can provide advice on lighting and contrast to maximize
remaining vision. These professionals also have access to non-visual aids, and can instruct
patients in their uses.
Once the emotional shock of the disability is overcome, if alternative techniques (basic
rehabilitation) are learnt, good quality of life and an adjustment to the disability can be
achieved, not only in the case of low vision, but also in the case of blindness.
According to an article published by The Academy of Psychosomatics Medicine, in a sample
of patients affected by progressive diabetic retinopathy, only those who had reached total
blindness actually displayed a decrease in psychic symptomatology, through learning
rehabilitation techniques. More marked distress remained in the subjects with persisting
partial sight. Unfulfilled expectations probably increased frustration at daily defeats, coupled
with fear of complete loss of residual sight. Acceptance of one's pathology and final outcome
is the basis for approaching and acquiring new behavioral patterns and creating good mental,
physical, and social equilibrium in those who become blind.
The subjects making the most use of rehabilitation instruments, who lived alone, and
preserved their own mobility and occupation were the least depressed, with the lowest risk of
suicide and the highest level of social integration.
Those with worsening sight and the prognosis of eventual blindness are at comparatively high
risk of suicide and thus may be in need of supportive services. These observations advocate
the establishment and extension of therapeutic and preventative programs to include patients
with impending and current severe visual impairment who do not qualify for services for the
blind. Ophthalmologists should be made aware of these potential consequences and
incorporate a place for mental health professionals in their treatment of these types of patients,
with a view to preventing the onset of depressive symptomatology, avoiding self-destructive
behavior, and improving the quality of life of these patients. Such intervention should occur in
the early stages of diagnosis, particularly as many studies have demonstrated how rapid
acceptance of the serious visual handicap has led to a better, more productive compliance with
rehabilitation programs. Moreover, psychological distress has been reported (and is
exemplified by our psychological autopsy study) to be at its highest when sight loss is not
complete, but the prognosis is unfavorable. Therefore, early intervention is imperative for
enabling successful psychological adjustment.
According to the Catalan Association for the Blind and Visually Impaired (ACCDV),
experience tells that seeking the support of other people affected is a good therapy to
overcome the disability, not only for the individual affected but for their families as well.
There are associations that give this kind of support and can put the person in touch with
professionals specialized in the collective's problems.
The Low Vision Examination
It is critical that all patients be examined by an optometrist or ophthalmologist specializing in
Low Vision Care prior to other rehabilitation training to rule out potential medical or surgical
correction for the problem and to establish a careful baseline refraction and prescription of
both normal and low vision glasses and optical aids. Only a doctor is qualified to evaluate
visual functioning of a compromised visual syetem effectively
Types of help available
The ACCDV states that medical help aside, the main ones are, in first place, information;
secondly, what help the administration offers; and finally the ones which facilitate personal
rehabilitation, education, and work and social integration.
Information is fundamental: doctors and sanitary personnel must have this information to
offer the patient when the moment is right. The desolation that doctors experience when they
must tell a patient they can't do anything more is only surpassed by the loneliness and
isolation the patient, who does not know where to go or what to do for help. Administrative
aids are valuable allies, though sometimes they may lie hidden under a legal mess. Adaptation
to the disability and psychological help are priority-one issues and must be confronted from
the start. Not least =important and almost as urgent is the education of the patient and their
family to confront the new situation. The adaptation of the work place (the one the person
currently has or a different one) is regulated by laws and norms and there are interesting
subventions for companies that make the necessary modifications to allow a person with
disabilities into their work force; therefore the reluctance to hire visually handicapped people
is an anti-economic prejudice, for the company and society. Lastly, social integration aids
facilitate adapted leisure and cultural activities, and private and public initiatives tending to
improve mobility and better access to information for everybody, including the visually
impaired.
Optical Aids
The vast majority of patients with low vision can be helped to function at a higher level with
the use of low vision devices. Low vision specialists recommend appropriate low vision
devices and counsel patients on how better to deal with their reduced vision in general. Many
government and private organizations exist to aid the visually impaired.
Improving Far sight: works best with static objects
•
•
•
•
•
TV
Theater
Cinema
Contemplating scenery
Seeing the bus number
Improving Near sight: the person must work closer to the object
•
•
•
Reading
Writing
Crafts
Improving sensitivity to contrast: the person must use special optical filters
Other tools:
•
•
•
Book stands
Special lights
Grid paper
•
•
Magnified games
Watches, audio thermometers, special phones, etc.
Effectivity of optical aids
In a study performed by this specialist on 1,000 patients, all subjects with a visual acuity
above 0.02 decimal (20/1000 feet) significantly improved their vision. From this group, 48%
were very satisfied with their visual aids, 44% were satisfied, 5% little satisfied and 3%
unsatisfied. Adaptation process to visual aids In the patient's first visit, the most adequate
options for their particular case are studied, taking into consideration their psychological,
cultural, social and work factors, and the degree of improvement experienced with the
selected aids, advising the patient on which aids will yield a better quality of life. After the
specific adaptation, there is a follow up to ensure the patient is correctly using and taking the
best advantage of the visual aids. In some cases (approx. 4%), the initial visual aids must be
changed. Once the patient is released, a report on their first visit and follow up is given to
their eye doctor or the professional who made the referral. We believe that low vision, as a
complementary technique to ophthalmology, has a great future, due to the progress of science,
the increase of life expectancy, and the increasing need people have to access information.
Other aids
For the totally blind, there are books in braille, audio-books, machines and computer
programs which transform text files into sound. low vision people can, of course, make use of
these tools as well.
Computers are, precisely, fundamental tools of integration for the visually impaired person.
They allow, using standard or specific programs, screen magnification and conversion of text
into sound or touch (Braille line), and are useful for all levels of visual handicap. OCR
scanners can, in conjunction with text-to-speech software, read the contents of books and
documents aloud via computer. Vendors also build closed-circuit televisions that
electronically magnify paper, and even change its contrast and color, for visually impaired
users. For more information, consult
Conclusions
An ever-increasing number of people are at risk of visual impairment as populations grow and
demographic shifts move towards the predominance of older age groups. Potentially blinding
eye conditions such as age-related macular degeneration (AMD), diabetic retinopathy and
glaucoma are increasing as the number of people affected grows. These are noncommunicable chronic eye diseases to which the principles of long-term care including issues
of cost of treatment and compliance (adherence) apply. Additionally, more programmes for
those with low vision will need to be made available.
http://en.wikipedia.org/wiki/Low_vision
VISUAL ACUITY
Visual acuity (VA) is acuteness or clearness of vision, especially form vision, which is
dependent on the sharpness of the retinal focus within the eye and the sensitivity of the
interpretative faculty of the brain.
VA is a quantitative measure of the ability to identify black symbols on a white background at
a standardized distance as the size of the symbols is varied. It is the most common clinical
measurement of visual function . In the term "20/20 vision" the numerator refers to the
distance in feet from which a person can reliably distinguish objects separated by an angle of
1 arc minute. The denominator is the distance related to a person with standard VA. The
metric equivalent is 6/6 vision
http://en.wikipedia.org/wiki/Visual_acuity
Traditional Snellen chart used for visual acuity testing.
To resolve detail, the eye's optical system has to project a focused image on the fovea, a
region inside the macula having the highest density of cone photoreceptors (the only kind of
photoreceptors existing on the fovea), thus having the highest resolution and best color vision.
Acuity and color vision, despite being done by the same cells, are different physiologic
functions that don't interrelate except by position. Acuity and color vision can be affected
independently.
Light travels from the fixation object to the fovea through an imaginary path called the visual
axis. The eye's tissues and structures that are in the visual axis (and also the tissues adjacent to
it) affect the quality of the image. These structures are: tear film, cornea, anterior chamber,
pupil, lens, vitreous, and finally the retina. The posterior part of the retina, called the retinal
pigment epithelium (RPE) is responsible for, among many other things, absorbing light that
crosses the retina so it cannot bounce to other parts of the retina. (However in many
vertebrates, such as cats, where high visual acuity is not a priority, there is a reflecting
tapetum layer that gives the photoreceptors a "second chance" to absorb the light, thus
improving the ability to see in the dark. This is what causes an animal's eyes to seemingly
glow in the dark when a light is shone on them.) The RPE also has a vital function of
recycling the chemicals used by the rods and cones in photon detection. If the RPE is
damaged and does not clean up this "shed" blindness can result.
Visual acuity is also affected by the size of the pupil. Optical aberrations of the eye that
decrease the sharpness of the image on the retina and hence visual acuity are at a maximum
when the pupil is largest (8 millimeters in diameter), as in low light conditions. On the other
hand, smear in image sharpness due to light wave diffraction is minimal, which would
increase acuity. However, optical aberrations dominate and acuity decreases somewhat with
large pupils. With tiny pupils (1-2 mm), optical aberrations decrease but diffraction increases
and dominates, again causing a modest decrease in acuity. Optimal pupil diameter for best
visual acuity in normal, healthy eyes tends to be in the middle, around 3 or 4 mm.
If the optics of the eye were otherwise perfect, theoretically acuity would be limited by pupil
diffraction to 0.4 minutes of arc (minarc) or 20/8 acuity. The smallest cone cells in the fovea
also have sizes corresponding to 0.4 minarc of the visual field, which also places a lower limit
on acuity. The optimal acuity of 0.4 minarc or 20/8 can be demonstrated using a laser
interferometer that bypasses any defects in the eye's optics and projects a pattern of dark and
light bands directly on the retina. Laser interferometers are now used routinely in patients
with optical problems, such as cataracts, to assess the health of the retina before subjecting
them to surgery.
The visual cortex is the part of the cerebral cortex in the posterior (occipital) part of the brain
responsible for processing visual stimuli. The central 10° of field (approximately the
extension of the macula) is represented by at least 60% of the visual cortex. Many of these
neurons are believed to be involved directly in visual acuity processing.
Proper development of normal visual acuity depends on an animal having normal visual input
when it is very young. Any visual deprivation, that is, anything intefering with such input
over a prolonged period, such as a cataract, severe eye turn or strabismus, or covering or
patching the eye during medical treatment, will usually result in a severe and permanent
decrease in visual acuity in the affected eye if not treated early in life. The decreased acuity is
reflected in various abnormalities in cell properties in the visual cortex. These changes
include a marked decrease in the number of cells connected to the affected eye as well as few
cells connected to both eyes, resulting in a loss of binocular vision and depth perception, or
stereopsis. The period of time over which an animal is highly sensitive to such visual
deprivation is referred to as the critical period.
The eye is connected to the visual cortex by the optic nerve coming out of the back of the eye.
The two optic nerves come together behind the eyes at the optic chiasm, where about half of
the fibers from each eye cross over to the opposite side and join fibers from other eye
representing the corresponding visual field, the combined nerve fibers from both eyes forming
the optic tract. This ultimately forms the physiological basis of binocular vision. The tracts
project to a relay station in the midbrain called the lateral geniculate nucleus and then to the
visual cortex along a collection of nerve fibers called the optic radiations.
Any pathological process in the visual system, even in older humans beyond the critical
period, will often cause decreases in visual acuity. Thus measuring visual acuity is a simple
test in accessing the health of the eyes, the visual brain, or pathway to the brain. Any
relatively sudden decrease in visual acuity is always a cause for concern. Common causes of
decreases in visual acuity are cataracts and scarred corneas, which affect the optical path,
diseases that affect the retina, such as macular degeneration and diabetes, diseases affecting
the optic pathway to the brain such as tumors and multiple sclerosis, and diseases affecting
the visual cortex such as tumors and strokes.
Visual acuity expression
Visual acuity scales
Foot Metre Decimal LogMAR
20/200 6/60
0.10
1.00
20/160 6/48
0.13
0.90
20/120 6/36
0.17
0.78
20/100 6/30
0.20
0.70
20/80 6/24
0.25
0.60
20/60 6/18
0.33
0.48
20/50 6/15
0.40
0.40
20/40 6/12
0.50
0.30
20/30 6/9
0.63
0.18
20/25 6/7.5 0.80
0.10
20/20 6/6
1.00
0.00
20/16 6/4.8 1.25
-0.10
20/12 6/3.6 1.67
-0.22
20/10 6/3
-0.30
2.00
Visual acuity is often measured according to the size of letters viewed on a Snellen chart or
the size of other symbols, such as Landolt Cs or Tumbling E.
In some countries, acuity is expressed as a vulgar fraction, and in some as a decimal number.
Using the foot as a unit of measurement, (fractional) visual acuity is expressed relative to
20/20. Otherwise, using the metre, visual acuity is expressed relative to 6/6. For all intents and
purposes, 6/6 vision is equivalent to 20/20. In the decimal system, the acuity is defined as the
reciprocal value of the size of the gap (measured in arc minutes) of the smallest Landolt C that
can be reliably identified. A value of 1.0 is equal to 20/20.
LogMAR is another commonly used scale which is expressed as the logarithm of the
minimum angle of resolution. LogMAR scale converts the geometric sequence of a traditional
chart to a linear scale. It measures visual acuity loss; positive values indicate vision loss,
while negative values denote normal or better visual acuity. This scale is rarely used
clinically; it is more frequently used in statistical calculations because it provides a more
scientific equivalent for the traditional clinical statement of “lines lost” or “lines gained”,
which is valid only when all steps between lines are equal, which is not usually the case.
A visual acuity of 20/20 is frequently described as meaning that a person can see detail from
20 feet away the same as a person with normal eyesight would see from 20 feet. If a person
has a visual acuity of 20/40, he is said to see detail from 20 feet away the same as a person
with normal eyesight would see it from 40 feet away. It is possible to have vision superior to
20/20: the maximum acuity of the human eye without visual aids (such as binoculars) is
generally thought to be around 20/10 (6/3) however, recent test subjects have exceeded 20/8
vision. Recent developments in optometry have resulted in corrective lenses conferring upon
the wearer a vision of up to 20/10. Some birds, such as hawks, are believed to have an acuity
of around 20/2; in this respect, their vision is much better than human eyesight.
When visual acuity is below the largest optotype on the chart, either the chart is moved closer
to the patient or the patient is moved closer to the chart until the patient can read it. Once the
patient is able to read the chart, the letter size and test distance are noted. If the patient is
unable to read the chart at any distance, he or she is tested as follows:
Name
Abbreviation
Counting Fingers CF
Hand Motion
HM
Light Perception LP
No Light
Perception
NLP
Definition
Ability to count fingers at a given distance.
Ability to distinguish a hand if it is moving or not in front
of the patient's face.
Ability to distinguish if the eye can perceive any light.
Inability to see any light. Total blindness.
Many humans have one eye that has superior visual acuity over the other. If a person cannot
achieve a visual acuity of 20/200 (6/60) or above in the better eye, even with the best possible
glasses, then that person is considered legally blind in the United States. A person with a
visual field narrower than 20 degrees also meets the definition of legally blind.
A person's visual acuity is registered documenting the following: whether the test was for
distant or near vision, the eye(s) evaluated and whether corrective lenses (i.e. spectacles or
contact lenses) were used:
•
•
•
•
Distance from the chart
o D (distant) for the evaluation done at 20 feet (or 6 meters).
o N (near) for the evaluation done at 15.7 inches (or 40 cm).
Eye evaluated
o OD (Latin oculus dexter) for the right eye.
o OS (Latin oculus sinister) for the left eye.
o OU (Latin oculi uterque) for both eyes.
Usage of spectacles during the test
o cc (Latin cum correctore) with correctors.
o sc: (Latin sine correctore) without correctors.
Pinhole
o PH abbreviation is used followed by the visual acuity measured with it.
So, distant visual acuity of 20/60 and 20/25 with pinhole in the right eye will be:
DscOD 20/60 PH 20/25
Distant visual acuity of count fingers and 20/50 with pinhole in the left eye will be:
DscOS CF PH 20/50
Near visual acuity of 20/25 with pinhole remaining at 20/25 in both eyes with spectacles will
be:
NccOU 20/25 PH 20/25
"Dynamic visual acuity" defines the ability of the eye to visually discern fine detail in a
moving object.
Measurement
Visual acuity is typically measured monocularly rather than binocularly with the aid of an
optotype chart for distant vision, an optotype chart for near vision, and an occluder to cover
the eye not being tested. The examiner may also occlude an eye by sliding a tissue behind the
patient's eyeglasses, or instructing the patient to use his or her hand. This latter method is
typically avoided in professional settings as it may inadvertently allow the patient to peek
through his or her fingers, or press the eye and alter the measurement when that eye is
evaluated.
1. Place the chart at 20 feet (or 6 meters) and illuminate to 480 lux at that distance.
2. If the patient uses glasses, then the test is performed using them.
3. Place the occluder in front of the eye that is not being evaluated. The first evaluated
eye is the one that is believed to see less or the one the patient says that is seeing less.
4. Start first with the big optotypes and proceed to the smaller ones. The patient has to
identify every one on the line being presented and communicate it to the physician.
5. If the measurement is reduced (below 20/20) then the test using a pinhole should be
done and register the visual acuity using the pinhole. Both measures should be
registered, with and without using pinhole.
6. Change the occluder to the other eye and proceed again from the 4th step.
7. After both eyes have been evaluated in distant visual acuity, proceed to evaluate near
visual acuity placing a modifid snellen chart for near vision (such as the Rosembaum
chart) at 15.7 inches (or 40 centimeters). Then repeat the test from the 2nd step.
In some cases, binocular visual acuity will be measured, because usually binocular visual
acuity is slightly better than monocular visual acuity.
Measurement considerations
Visual acuity measurement involves more than being able to see the optotypes. The patient
should be cooperative, understand the optotypes, be able to communicate with the physician,
and many more factors. If any of these factors is missing, then the measurement will not
represent the patient's real visual acuity.
Visual acuity is a subjective test meaning that if the patient is unwilling or unable to
cooperate, the test cannot be done. A patient being sleepy, intoxicated, or having any disease
that can alter the patient's consciousness or his mental status can make the measured visual
acuity worse than it actually is.
Illiterate patients who cannot read letters and/or numbers will be registered as having very
low visual acuity if this is not known. Some of the patients will not tell the physician that they
don't know the optotypes unless asked directly about it. Brain damage can result in a patient
not being able to recognize printed letters, or being unable to spell them.
A motor inability can make a person respond incorrectly to the optotype shown and
negatively affect the visual acuity measurement.
Variables such as pupil size, background adaptation luminance, duration of presentation, type
of optotype used, interaction effects from adjacent visual contours (or “crowding") can all
affect visual acuity measurement.
Visual acuity testing in children
The newborn’s visual acuity is approximately 20/400, developing to 20/20 by two years. [8]
The measurement of visual acuity in infants, pre-verbal children and special populations (for
instance, handicapped individuals) is not always possible with a letter chart. For these
populations, specialised testing is necessary. As a basic examination step, one must check
whether visual stimuli can be fixed, centered and followed.
More formal testing using preferential looking techniques use Teller acuity cards (presented
by a technician from behind a window in the wall) to check if the child is more visually
attentive to a random presentation of vertical or horizontal bars on one side compared with
blank page on the other side - the bars become progressively finer or closer together, and the
endpoint is noted when the child in its adult carer's lap equally prefers the two sides.
Another popular technique is electro-physiologic testing using visual evoked potentials
(VEP), which can be used to estimate visual acuity in doubtful cases and expected severe
vision loss cases like Leber's congenital amaurosis.
VEP testing of acuity is somewhat similar to preferential looking in using a series of black
and white stripes or checkerboard patterns (which produce larger responses than stripes).
However, behaviorial responses are not required. Instead brain waves created by the
presentation of the patterns are recorded. The patterns become finer and finer until the evoked
brain wave just disappears, which is considered to be the endpoint measure of visual acuity. In
adults and older, verbal children capable of paying attention and following instructions, the
endpoint provided by the VEP corresponds very well to the perceptual endpoint determined
by asking the subject when they can no longer see the pattern. There is an assumption that this
correspondence also applies to much younger children and infants, though this doesn't
necessarily have to be the case. Studies do show the evoked brain waves, as well as derived
acuities, are very adult-like by one year of age.
For reasons not totally understood, until a child is several years old, visual acuities from
behavioral preferential looking techniques typically lag behind those determined using the
VEP, a direct physiological measure of early visual processing in the brain. Possibly it takes
longer for more complex behavioral and attentional responses, involving brain areas not
directly involved in processing vision, to mature. Thus the visual brain may detect the
presence of a finer pattern (reflected in the evoked brain wave), but the "behavioral brain" of a
small child may not find it salient enough to pay special attention to.
A simple but less-used technique is checking oculomotor responses with an optokinetic
nystagmus drum, where the subject is placed inside the drum and surrounded by rotating
black and white stripes. This creates an involuntary flicking or nystagumus of the eyes as they
attempt to track the moving stripes. There is a good correspondence between the optikinetic
and usual eye-chart acuities in adults. A potentially serious problem with this technique is that
the process is reflexive and mediated in the low-level brain stem, not in the visual cortex.
Thus someone can have a normal optokinetic response and yet be cortically blind with no
conscious visual sensation.
"Normal" vision
Visual acuity depends upon how accurately light is focused on the retina (mostly the macular
region), the integrity of the eye's neural elements, and the interpretative faculty of the brain.
"Normal" visual acuity is frequently considered to be what was defined by Snellen as the
ability to recognize an optotype when it subtended 5 minutes of arc, that is Snellen's chart
20/20 feet, 6/6 meter, 1.00 decimal or 0.0 logMAR. In humans, the maximum acuity of a
healthy, emmetropic eye (and even ametropic eyes with correctors) is approximately 20/16 to
20/12 so it is inaccurate to refer to 20/20 visual acuity as "perfect" vision. 20/20 is the visual
acuity needed to discriminate two points separated by 1 arc minute -- about 1/16 of an inch at
20 feet. This is because a 20/20 letter, E for example, has three limbs and two spaces in
between them, giving 5 different detailed areas. The ability to resolve this therefore requires
1/5 of the letters total arc, which in this case would be 1 minute. The significance of the 20/20
standard can best be thought of as the lower limit of normal or as a screening cutoff. When
used as a screening test subjects that reach this level need no further investigation, even
though the average visual acuity of healthy eyes is 20/16 to 20/12
Some people may suffer from other visual problems, such as color blindness, reduced
contrast, or inability to track fast-moving objects and still have normal visual acuity. Thus,
normal visual acuity does not mean normal vision. The reason visual acuity is very widely
used is that it is a test that corresponds very well with the normal daily activities a person can
handle, and evaluate their impairment to do them.
Other measures of visual acuity
Normally visual acuity refers to the ability to resolve two separated points or lines, but there
are other measures of the ability of the visual system to discern spatial differences.
Vernier acuity measures the ability to align two line segments. Humans can do this with
remarkable accuracy. Under optimal conditions of good illumination, high contrast, and long
line segments, the limit to vernier acuity is about 8 arc seconds or 0.13 arc minutes, compared
to about 0.6 arc minutes (20/12) for normal visual acuity or the 0.4 arc minute diameter of a
foveal cone. Because the limit of vernier acuity is well below that imposed on regular visual
acuity by the "retinal grain" or size of the foveal cones, it is thought to be a process of the
visual cortex rather than the retina. Supporting this idea, vernier acuity seems to correspond
very closely (and may have the same underlying mechanism) enabling one to discern very
slight differences in the orientations of two lines, where orientation is known to be processed
in the visual cortex.
The smallest detectable visual angle produced by a single fine dark line against a uniformally
illuminated background is also much less than foveal cone size or regular visual acuity. In this
case, under optimal conditions, the limit is about 0.5 arc seconds, or only about 2% of the
diameter of a foveal cone. This produces a contrast of about 1% with the illumination of
surrounding cones. The mechanism of detection is the ability to detect such small differences
in contrast or illumination, and does not depend on the angular width of the bar, which cannot
be discerned. Thus as the line gets finer, it appears to get fainter but not thinner.
Stereoscopic acuity is the ability to detect tiny differences in depth with the two eyes. For
more complex targets, stereoacuity is similar to normal monocular visual acuity, or around
0.6-1.0 arc minutes, but for much simpler targets, such as vertical rods, may be as low as only
2 arc seconds. Although stereoacuity normally corresponds very well with monocular acuity,
it may be very poor or even absent even with normal monocular acuities. Such individuals
typically have abnormal visual development when they are very young, such as an alternating
strabismus or eye turn, where both eyes rarely or never point in the same direction and
therefore do not function together.
http://en.wikipedia.org/wiki/Visual_acuity
GLARE
Glare is difficulty seeing in the presence of bright light such as direct or reflected sunlight or
artificial light such as car headlamps at night. Because of this, some cars include mirrors with
automatic anti-glare functions.
Glare can reduce visibility by:
•
•
•
•
•
reduction of brightness of the rest of the scene by constriction of the pupils
reduction in contrast of the rest of the scene by scattering of the bright light within the
eye.
reduction in contrast by scattering light in particles in the air, as when the headlights
of a car lights up the fog close to the vehicle, impeding vision at larger distance.
reduction in contrast between print and paper by reflection of the light source in the
printed matter (veiling glare).
reduction in contrast by reflection of bright areas on the surface of a transparent
medium as glass, plastic or water; for example when the sky is reflected in a lake, so
that the bottom below or objects in the water cannot be seen (veiling glare).
Sunglasses are often worn to reduce glare; polarized sunglasses are designed to reduce glare
caused by light reflected from non-metallic surfaces such as water, glossy printed matter or
painted surfaces.
http://en.wikipedia.org/wiki/Glare_%28vision%29
Lens flare is the light scattered in lens systems through generally unwanted image formation
mechanisms, such as internal reflection and scattering from material inhomogeneities in the
lens. These mechanisms differ from the intended image formation mechanism that depends on
refraction of the image rays. For good optical systems and most images, flare is a secondary
effect that is widely distributed across the image and thus not visible. But when an image
includes a very bright light source, flare generated by a bright image region can have enough
intensity to become very visible. The light produced by flare mechanisms superimposes
broadly across the image, adding light to dark image regions and reducing image contrast.
Lenses with large numbers of elements such as zooms tend to exhibit greater lens flare, as
they contain multiple surfaces at which unwanted internal scattering occurs.
The spatial distribution of the lens flare typically manifests as several starbursts, rings, or
circles in a row across the image or view. Lens flare patterns typically spread widely across
the scene and change location with the camera's movement relative to light sources, tracking
with the light position and fading as the camera points away from the bright light until it
causes no flare at all. The specific spatial distribution of the flare depends on the shape of the
aperture of the image formation elements. For example, if the lens has a 6-bladed aperture, the
flare may have a hexagonal pattern.
Such internal scattering is also present in the human eye and manifests in an unwanted veiling
glare that is apparent when viewing very bright lights or highly reflective (e.g. specular)
surfaces.
When a bright light source is shining on the lens but not in its field of view, lens flare appears
as a haze that washes out the image and reduces contrast. This can be avoided by shading the
lens (the purpose for which lens hoods are designed). In a studio, a gobo or set of barn doors
can be attached to the lighting to keep it from shining on the camera. Modern lenses use lens
coatings to reduce the amount reflection and minimize flare.
Deliberate use
A lens flare is often deliberately used to invoke a sense of drama. A lens flare is also useful
when added to an artificial or modified image composition because it adds a sense of realism,
implying that the image is an un-edited original photograph of a "real life" scene.
For both of these reasons (implying realism and/or drama) artificial lens flare is a common
effect in various graphics editing programs, although its use can be a point of contention
among professional graphic designersLens flare was one of the first special effects developed
for computer graphics because it is the result of relatively simple optical principles. During
the mid- to late-1990s, it was a popular graphical effect for computer and video games, and is
now accompanied by other more complex atmospheric effects that add a greater sense of
realism
http://en.wikipedia.org/wiki/Lens_flare
SLEEP DISORDER
A sleep disorder (somnipathy) is a medical disorder of the sleep patterns of a person or
animal. Some sleep disorders are serious enough to interfere with normal physical, mental and
emotional functioning. A test commonly ordered for some sleep disorders is the
polysomnogram.
Common sleep disorders
The most common sleep disorders include:
•
•
Bruxism: The sufferer involuntarily grinds or clenches his or her teeth while sleeping.
Delayed sleep phase syndrome (DSPS): A sleep disorder of circadian rhythm,
characterized by the inability to wake up and fall asleep at the desired times, but not
by inability to stay asleep.
•
•
•
•
•
•
•
•
•
•
•
•
Hatzfeldt Syndrome or Systemic Neuro-Epiphysial Disorder (SNED) is a somnipathy
mainly characterized by an irregular sleep pattern, as well as irregular behavior.
Hypopnea syndrome: Abnormally shallow breathing or slow respiratory rate while
sleeping.
Narcolepsy: The condition of falling asleep spontaneously and unwillingly at
inappropriate times.
Night terror or Pavor nocturnus or sleep terror disorder: abrupt awakening from sleep
with behavior consistent with terror.
Parasomnias: Include a variety of disruptive sleep-related events.
Periodic limb movement disorder (PLMD): Sudden involuntary movement of arms
and/or legs during sleep, for example kicking the legs. Also known as nocturnal
myoclonus. See also Hypnic jerk, which is not a disorder.
Rapid eye movement behavior disorder (RBD): Acting out violent or dramatic dreams
while in REM sleep.
Restless legs syndrome (RLS): An irresistible urge to move legs. RLS sufferers often
also have PLMD.
Shift work sleep disorder (SWSD).
Sleep apnea: The obstruction of the airway during sleep, causing loud snoring and
sudden awakenings when breathing stops.
Sleep paralysis is characterized by temporary paralysis of the body shortly before or
after sleep. Sleep paralysis may be accompanied by visual, auditory or tactile
hallucinations.
Sleepwalking or somnambulism: Engaging in activities that are normally associated
with wakefulness (such as eating or dressing), which may include walking, without the
conscious knowledge of the subject.
Broad classifications of sleep disorders
•
•
Dysomnias - A broad category of sleep disorders characterized by either
hypersomnolence or insomnia. The three major subcategories include intrinsic (i.e.,
arising from within the body), extrinsic (secondary to environmental conditions or
various pathologic conditions), and disturbances of circadian rhythm. MeSH
o Insomnia
o Narcolepsy
o Obstructive sleep apnea
o Restless leg syndrome
o Periodic limb movement disorder
o Hypersomnia
Recurrent hypersomnia - including Kleine-Levin syndrome
Posttraumatic hypersomnia
"Healthy" hypersomnia
o Circadian rhythm sleep disorders
Delayed sleep phase syndrome
Advanced sleep phase syndrome
Non-24-hour sleep-wake syndrome
Parasomnias
o REM sleep behaviour disorder
o Sleep terror
o Sleepwalking (or somnambulism)
o Bruxism (Tooth-grinding)
Bedwetting or sleep enuresis.
Sudden infant death syndrome (or SIDS)
Sleep talking (or somniloquy)
Sleep sex (or sexsomnia)
Exploding head syndrome - Waking up in the night hearing loud noises.
Medical or Psychiatric Conditions that may produce sleep disorders
o Psychoses (such as Schizophrenia)
o Mood disorders
o Depression
o Anxiety
o Panic
o Alcoholism
Sleeping sickness - can be carried by the Tsetse fly
Snoring - Not a disorder in and of itself, but it can be a symptom of deeper problems.
o
o
o
o
o
•
•
•
Common causes of sleep disorders
Changes in life style, such as shift work change (SWC), can contribute to sleep disorders.
Other problems that can affect sleep:
•
•
•
•
•
•
•
•
•
•
Anxiety
Back pain
Chronic pain
Sciatica
Neck pain
Environmental noise
Incontinence
Various drugs - Many drugs can affect the ratio of the various stages of sleep, thus
affecting the overall quality of sleep. Poor sleep can lead to accumulation of Sleep
debt.
Endocrine imbalance mainly due to Cortisol but not limited to this hormone. Hormone
changes due to impending menstruation or during the menopause transition years.
Chronobiological disorders, mainly Circadian rhythm disorders
A sleep diary can be used to help diagnose, and measure improvements in, sleep disorders.
The Epworth Sleepiness Scale and the Morningness-Eveningness Questionnaire
According to Dr. William Dement, of the Stanford Sleep Center, anyone who snores and has
daytime drowsiness should be evaluated for sleep disorders.
Any time back pain or another form of chronic pain is present, both the pain and the sleep
problems should be treated simultaneously, as pain can lead to sleep problems and vice versa.
General Principles of Treatment
Treatments for sleep disorders generally can be grouped into three categories:
•
•
behavioral/ psychotherapeutic treatments,
medications, and
•
other somatic treatments.
None of these general approaches is sufficient for all patients with sleep disorders. Rather, the
choice of a specific treatment depends on the patient's diagnosis, medical and psychiatric
history, and preferences, as well as the expertise of the treating clinician. In general,
medications and somatic treatments provide more rapid symptomatic relief from sleep
disturbances. On the other hand, some emerging evidence suggests that treatment gains with
behavioral treatment of insomnia may be more durable than those obtained with medications.
Some sleep disorders, such as narcolepsy, are best treated pharmacologically, whereas others,
such as chronic and primary insomnia, are more amenable to behavioral interventions. The
management of sleep disturbances that are secondary to mental, medical, or substance abuse
disorders should focus on the underlying conditions.
For most sleep disorders, behavioral/psychotherapeutic and pharmacological approaches are
not incompatible and can be effectively combined to maximize therapeutic benefits.
http://en.wikipedia.org/wiki/Sleep_disorder
OPTOMETRY
Optometry is a doctoral-degree health care profession concerned with eyes and related
structures, as well as vision, visual systems, and vision information processing in humans.
Optometrists are the primary eye and vision health care providers in the United States.
Like most health professions, optometry education, certification, and practice is regulated in
most countries. Optometrists and optometry-related organizations interact with governmental
agencies, other health care professionals, and the community to deliver eye and vision care.
Optometry is one of two doctoral-degree professional eye care professions, the other being
ophthalmology.
Scope of practice
Optometrists are primary health care providers for the eye and visual system. Optometrists are
also known as doctors of optometry. They examine, diagnose, and medically treat eye
diseases, injuries, and disorders of the eyes and visual system, including refractive problems
such as near- or far-sightedness, and identify related systemic medical conditions affecting the
eyes and ocular adnexa. In some locations, optometrists may perform laser surgery.
The practice is defined by the World Council of Optometry (a member of the World Health
Organisation) as follows:
Optometry is a healthcare profession that is autonomous, educated, and regulated
(licensed/registered), and optometrists are the primary healthcare practitioners of the
eye and visual system who provide comprehensive eye and vision care, which includes
refraction and dispensing, detection/diagnosis and management of disease in the eye,
and the rehabilitation of conditions of the visual system.
Optometrists may serve the general public; specialize in work with the elderly, children, or
partially-sighted persons who need specialized visual devices; develop and implement ways to
protect workers eyes from on-the-job strain or injury; or specialize in contact lenses, sports
vision, or vision therapy.
Eye and vision examination
As with most health care, examination often includes history-taking of both eye-related health
and optical and visual functioning-related aspects of the patient. The typical examination has
two components: the evaluation of the health status for the detection of eye disease, and
evaluating the optical and vision characteristics of the eye and observations during testings.
Examination of ocular health may include:
•
•
•
•
•
•
inspection of the external structures of the eye such as Cornea, Anterior Chamber,
Physiological Lens as well as internal ocular structures such as Retina and Optic
Nerve. This is done with various specialty equipment
observation of various eye movements and alignment
observation of pupillary reaction to light as a neurological test
observation of overall health status of adnexal ocular structures such as eyelids and
eyelashes, as well as the lacrimal system among others
measurement of eye pressure also know as intraocular pressure
evaluation of functional aspects of the eye such as visual fields
Examination of vision and visual function may include:
•
•
•
•
measurement of vision at distance and near
detailed refraction for distance and near with specialty equipment
measurement of optical aids such as glasses, contact lenses and magnifiers
measurement of stereopsis, color vision screening tests
Examination of visual skills:
•
applying a battery of structured visual tasks for patient to complete to evaluate the
functional characteristics of the visual system such as tracking and focusing aspects as
well as muscle coordination.
Pre-optometric education
Prerequisites for admission to optometry schools are the same as admission to medical,
osteopathic, and dental programs.
Optometric education
Optometrists complete a 4-year program that leads to a Doctorate in Optometry (O.D.)
degree. Many optometrists complete a one- or two-year residency to specialize.
Examples of equipment used for eye and vision health testing
Mypes of equipment are used during an eye examination. Vision charts and machines are used
to measure vision and visual fields. Trial (spectacle and contact) lenses or a phoropter and
retinoscope may be used during refraction. Prism bars, small objects, and occluders may be
used to assess eye movements and eye alignment. Test booklets, sheets, instructions, and
pencils may be used for visual information processing examination.
Penlights and transilluminators can be used when assessing pupil light response, a
neurological screening test. Specialty magnifiers, such as ophthalmoscopes and slit-lamp biomicroscopes, help with detailed inspection of external and internal anatomical ocular
structures. Diagnostic eye drops may also be used to assess the various anatomical structures
of the eyes.
Many optometrists use computerized equipment specifically designed to help diagnose and/or
monitor certain ocular diseases. For example, many optometrists' offices have various visual
field analyzers and tonometers that are helpful in diagnosing disease entity in early stages.
Optometrists use digital imaging equipment, such as digital cameras to document appearance
of the anterior and posterior parts of the eye. Corneal topographers are used to gather
information on anterior aspects of the anatomy of the eye and cornea. Other sophisticated
equipment such as Optical coherence tomography, GDX, or HRT II can be used for various
disease testing and treatment.
Diagnoses
Diagnoses made by optometry depends on integrating eye examination information.
Some ocular diseases can be associated with systemic, neural, or other disease complications.
Some ocular disorders may be treated by an optometrist. In many cases, referral to an
ophthalmologist may be required for surgical treatment.
Visual dysfunctions assessed by optometrists may include:
•
•
refractive error such as myopia, hyperopia, astigmatism and presbyopia
accommodative-vergence disorders (related to dynamic focus and eye alignment)
Common examples of ocular pathologies diagnosed and treated by optometrists include:
•
•
•
•
•
glaucoma
cataracts
infection and inflammation of the ocular surface including conditions of the cornea,
conjunctiva as well as internal pathologies of the retina, vitreous and optic nerve
among others
Strabismus (squint or turned eye) which might require surgical repair by an
ophthalmologist
Haemorrhage of vascular supply to the eye (internal or external)
Common examples of diseases of systemic origin with eye complications that can be
recognized and managed by evaluation of the ocular structures include:
•
•
•
diabetic eye disease and retinopathy caused by diabetes
retinal changes caused by other systemic disorders such as hypertension and
cholesterol problems
evaluation of ocular changes caused by medications such as oral contraceptives and
Plaquenil among others
Patient management
Optometry clinical patients management can include:
•
•
•
•
•
•
Counsel on status regarding comprehensive or detailed evaluations of the human eye.
Diagnosis and treatment or management of eye disease, ocular findings or visual
disturbance.
Prescribing medications such as antibiotics, antiinflammatory and other for the
treatment of eye conditions and diseases.
Prescribing optical aids such as glasses, contact lenses, magnifiers.
Prescribing low vision rehabilitation.
Prescribing vision therapy.
They advice and follow-up care regarding use of optical aids (especially contact lenses),
provide referral to other health professionals including internist and other primary care
physicians and particularly sub specialists like ophthalmologists for surgical consultation, and
interact with opticians and the optical industry, which manufacture the optical aids such as
glasses in accordance to optical prescriptions.
History
Optometric history is tied to the development of
•
•
•
•
vision science (related areas of medicine, microbiology, neurology, physiology,
psychology, etc)
optics, optical aids
optical instruments, imaging techniques
other eye care professions
The term optometrists was coined by Landolt in 1886, referring to the "fitting of glasses".
Prior to this, there was a distinction between "dispensing" and "refracting" opticians in the
19th century. The latter were later called optometrists.
Apparently the first schools of optometry were established in 1850-1900 (presumably in
USA) and contact lenses were first used in 1940s
Licensing
Most countries have regulations concerning optometry education and practice. Optometrists
like many other health care professionals are required to participate in ongoing continuing
education courses to stay current on the latest standards of care.
Optometry is officially recognized:
•
•
•
•
•
•
in North America (Canada and US)
in Latin America and some Caribbean countries
in most English speaking countries including UK, Republic of Ireland and Australia
in Europe including Spain, Germany and France
in Asia including Malaysia, China, Hong Kong, Thailand and Taiwan
in the Middle East including Saudi Arabia, Iran and Israel
United Kingdom
In the United Kingdom, optometrists have to complete a 3 or 4 year undergraduate honours
degree followed by a minimum of a one-year "pre-registration period" where they complete
supervised practice under the supervision of an experienced qualified practitioner. During this
year the pre-registration candidate is given a number of quarterly assessments and on
successfully passing all of these assessments, a final one-day set of examinations. Following
successful completion of these assessments and having completed one year's supervised
practice, the candidate qualifies for membership of The College of Optometrists and is
eligible to register as an optometrist with the General Optical Council (GOC).
There are 6 universities which offer Optometry in England, they are: Anglia Ruskin
University Aston University Bradford University Cardiff University London City Manchester
University
Registration with the GOC is mandatory to practice in the UK. Members of the College of
Optometrists may use the suffix MCOptom. Optometrists in the United Kingdom, as in most
countries except the United States and Canada, receive a Bachelor of Optometry or Masters
degree. They are not called "doctor" in the United Kingdom.
Europe
Currently, optometry education and licencing varies through out Europe. For example, in
Germany, the tasks of an optometrist are split between ophthalmologists and professionally
trained and certified opticians. In France, there is no regulatory framework and optometrists
are sometimes trained by completing an apprenticeship at an ophthalmologists' private office.
Since the formation of the European Union, "there exists a strong movement, headed by the
Association of European Schools and Colleges of Optometry (AESCO), to unify the
profession by creating a European-wide examination for optometry" and presumably also
standardised practice and education guidelines within EU countries.
Ireland
The profession of Optometry has been represented for over a century by the Association of
Optometrists, Ireland [AOI]. In Ireland an optometrist must first complete a four year degree
in Optometry at D.I.T. Kevin Street. Following successful completion of the degree, an
optometrist must then complete Professional Qualifying Examinations in order to be entered
into the register of the Opticians Board [Bord na Radharcmhaistoiri]. It is illegal to practice as
an optometrist in the Republic of Ireland, unless registered with the Board.
The A.O.I. runs a comprehensive continuing education and professional development
program on behalf of Irish optometrists. Unfortunately for the profession in Ireland, the
legislation governing Optometry was drafted in 1956 and is hopelessly out of date. The
unnecessary restrictions in this fifty year old piece of legislation restricts optometrists from
using their full range of skills, training and equipment for the benefit of the Irish public. The
amendment to the Act in 2003 addressed one of the most egregious restrictions - the use of
cycloplegic drugs to examine children. Review of this legislation is urgent to allow the public
to benefit from the skills of optometrists to reduce the enormous waiting lists in the public
health service particularly for children.
Distinction from ophthalmology
Ophthalmologists after obtaining a 4 year bachelors degree, attend medical school for 4 years
of medical training to obtain an MD degree. Ophthalmologists train for an additional three to
four years of residency training. Residency training in ophthalmology encompasses all aspects
of diagnosis and management of diseases that affect the eye, orbit, and neurological system of
the brain. This includes surgical treatment. Many ophthalmologists pursue additional
fellowship training in various subspecialties.
(Most of the following information pertains to Optometry in the United States): Optometrists
also acquire a 3-4 year bachelor degree followed by 4 years of Optometry school to earn an
OD or Doctor of Optometry degree. While in school, optometry students undergo internship
training and after completion of the degree, have options of 1 to 2 year residency programs for
further specialization.
Optometrists having completed a residency can further specialize in a particular area such as
Pediatric Optometry, Geriatric Optometry, Behavioral Optometry or Neuro-optometry.
Optometry school is more specialized in scope, with courses that include vision sciences,
health sciences, pharmacology, and clinical education. Examples include courses in visual
psychophysics, optics, as well as training in aspects of functional vision such as vision
therapy, binocular vision, and low vision. Optometrists are also trained extensively in
anatomy, histology, neurology, vision perception. They have a broad understanding of disease
etiology, management, and treatment. In general, optometrists can do the same things
ophthalmologists do with the exceptions of internal surgery and particular diseases (e.g. wet
macular degeneration, proliferative diabetic retinopathy, glaucoma surgery, cataracts) that
require extensive surgical expertise. Depending on state law, however, many optometrists are
licensed to perform minor surgery as well as laser surgery. Some states limit the prescribing
of oral medications by optometrists depending on licensure and regulatory requirements.
The two fields often have a mutually beneficial relationship:
Ophthalmologists may refer patients to optometrists for contact lenses or for optical aids or
low vision rehabilitation whilst continuing to treat the underlying disease/condition that may
have reduced vision. Similarly, complicated and emergency eye conditions are referred from
Optometry to Ophthalmology.
Both optometrists and ophthalmologists perform screening for common ocular problems
affecting children (i.e., amblyopia and strabismus) and the adult population (cataract,
glaucoma, and diabetic retinopathy).
Optometrists generally manage treatment of strabismus and amblyopia with vision therapy
while Ophthalmologists manage these disorders with refractive, orthoptic, medical and
surgical therapy.
Sub-specialties
There are currently nine sub-specialty residencies offered by various schools of optometry in
the United States:
1. Cornea and contact lenses
2. Family practice optometry
3. Geriatric optometry
4. Glaucoma
5. Low vision rehabilitation
6. orthoptic pratice
7. Ocular disease
8. Pediatric optometry
9. Primary care
10. Vision therapy and rehabilitation
Many of these sub-specialties are also recognised in other countries.
Please note, refractive surgery and ocular surgery fellowships involve learning how to comanage patients before and after eye surgery. Similarly, ocular disease residencies involve comanagement practice with other health professionals. Also the College of Optometrists in
Vision Development provides certification for eye doctors in vision therapy, behavioral and
developmental vision care, and "visual rehabilitation". Training in binocular vision and
orthoptics sub-specialties are often integrated into either pediatric or vision therapy programs.
http://en.wikipedia.org/wiki/Optometry
EYE CARE PROFESSIONAL
An eye care professional is an individual who provides a service related to the eyes or vision.
It is a general term that can refer to any healthcare worker involved in eye care, from one with
a small amount of post-secondary training to practitioners with a doctoral level of education.
Types of eye care professionals
•
•
Optometrist - A Doctor of Optometry (OD) trained to diagnose and treat common eye
diseases and disorders as well as refractive vision correction. In the US, the standard
education is four years of college, four years of Optometry school at an accredited
Doctor of Optometry program. An additional one to two years of residency, internship,
and/or fellowship and specialty training is required for specialty training.
Ophthalmologist - A Doctor of Medicine (MD) who specializes in surgical eye care.
In the US, this often requires four years of college, four years of medical school, and
four to six more years of residency, internship, and/or fellowship and sub specialty
training.
•
•
•
•
•
•
•
•
Ophthalmic Medical Practitioner - A Doctor of Medicine (MD) who specialises in
ophthalmic conditions but who has not completed a specialisation in Ophthalmology.
(term used in the UK).
Oculist - Older term for either an ophthalmologist or optometrist.
Ocularist - Specialize in the fabrication and fitting of ocular prostheses for people who
have lost eyes due to trauma or illness.
Optician - Specializes in the fitting and fabrication of ophthalmic lenses, spectacles,
contact lenses, low vision aids and ocular prosthetics. They may also be referred to as
an Optical Dispenser, Dispensing Optician, Ophthalmic Dispenser. The prescription
for the corrective lenses must be supplied by an ophthalmologist or optometrist. This
is a regulated profession in most jurisdictions.
Orthoptist - Specializes in ocular motility, which is the movement of the eye
controlled by the extraocular muscles.
Vision therapist - Works with patients that require vision therapy, such as low vision
patients.
Ophthalmic Medical Personnel - A collective term for allied health personnel in
ophthalmology. It is often used to refer to specialized personnel (unlike ocularists or
opticians). The Joint Commission on Allied Health Personnel in Ophthalmology
administers OMP certifications.
Optometric physician or medical optometrist - a term used by some optometrist that
denotes expanded licensure.
The distinction between optometrist and ophthalmologist
An optometrist is defined by the World Council of Optometry (a member of the World Health
Organisation) as follows:
Optometry is a healthcare profession that is autonomous, educated, and regulated
(licensed/registered), and optometrists are the primary healthcare practitioners of the eye and
visual system who provide comprehensive eye and vision care, which includes refraction and
dispensing, detection/diagnosis and management of disease in the eye, and the rehabilitation
of conditions of the visual system.
The American Academy of Ophthalmology describes an ophthalmologist as follows:
A medical doctor who specializes in all aspects of eye care including diagnosis, management,
and surgery of ocular diseases and disorders.
Two important distinctions are evident in these definitions. First, ophthalmologists are
medical doctors and have attended medical school and specialize in surgical care of the eye,
while optometrists are doctors of optometry who have attended optometry school and
specialize in the general care of the eye and vision. Second, ophthalmologists are responsible
for surgical treatment or diseases and disorders. Optometrists "provide comprehensive eye
and vision care, which includes refraction and detection/diagnosis and management of disease
in the eye" with limited surgical involvement.
There are also important similarities. Both optometrists and ophthalmologists treat patients
with medications and optical aids. Both perform screenings for common ocular problems
affecting children (such as amblyopia and strabismus) and the adult population (such as
cataracts, glaucoma, and diabetic retinopathy). Optometrists usually refer to ophthalmologists
for further assessment for surgical treatment of ocular diseases. Both are required to
participate in ongoing continuing education courses to maintain licensure and stay current on
the latest standards of care.http://en.wikipedia.org/wiki/Eye_care_professional
SIGNS OF EYE PROBLEMS IN ADULTS
Any changes in the appearance of your eyes or vision should be investigated further. Some
examples include:
•
Unusual trouble adjusting to dark rooms;
•
Difficulty focusing on near or distant objects;
•
Squinting or blinking due to unusual sensitivity to light or glare;
•
Change in color of iris;
•
Red-rimmed, encrusted or swollen lids;
•
Recurrent pain in or around eyes;
•
Double vision;
•
Dark spot at the center of viewing;
•
Lines and edges appear distorted or wavy;
•
Excess tearing or "watery eyes";
•
Dry eyes with itching or burning; and
•
Seeing spots, ghost-like images.
The following may be indications of potentially serious problems that might require
emergency medical attention:
•
Sudden loss of vision in one eye;
•
Sudden hazy or blurred vision;
•
Flashes of light or black spots;
•
Halos or rainbows around light;
•
Curtain-like blotting out of vision; and
•
Loss of peripheral (side) vision.
http://www.nlm.nih.gov/medlineplus/retinaldisorders.html#cat5
VISUAL SKILLS
Visual skills can be divided to into two main categories: visual perceptual motor skills and
ocular motor skills. Many of these visual skills are developed post-natally and often involve
processing visual (sight) and other sensory input.
Visual perceptual motor skills
Visual perceptual motor skills involve processing and using visual information. These skills
also help with planning and guiding eye/body movements.
•
•
•
•
•
•
visual memory (eg recall visual information in chunks or in spatial/temporal sequence)
visual spatial (eg mapping locations, direction concepts)
visual analysis (eg matching, discriminating, identifying)
visual motor integration (eg hand-eye coordination, visually guided mobility)
visual auditory integration (eg matching sounds with image or symbol, decoding &
encoding auditory to visual information)
visualization (eg manipulation - can imagine flips & rotations or image, other)
Ocular motor skills
Ocular motor skills include control of eye movements, fixations (looking at something at
specific location in space) and focus.
•
•
•
eye teaming (vergence - convergence, divergence)
eye focusing (accommodation)
eye movements & tracking (saccades & pursuits, eye movements for reading)
http://en.wikipedia.org/wiki/Visual_skills
Rehabilitation-counseling
Rehabilitation Counseling is focused on helping people who have disabilities achieve their
personal, career, and independent living goals through a counseling process. Rehabilitation
Counselors can be found in private practice, in rehabilitation facilities, universities, schools,
government agencies, insurance companies and other organizations where people are being
treated for congenital or acquired disabilities with the goal of going to or returning to work.
Education, Training & Certification
Education & Training In order to be certified, Rehab Counselors must obtain a Masters
Degree. The Council on Rehabilitation Education accredits qualifying institutions.
•
Length of education: Rehabilitation counselor education programs typically provide
between 18 and 24 months of academic and field-based clinical training. Clinical
training consists of a practicum and a minimum of 600 hours of supervised internship
•
•
experience. Clinical field experiences are available in a variety of community, state,
federal, and private rehabilitation-related programs.
Prerequisites: Although no formal requirements exist, most rehabilitation counseling
graduate students have undergraduate degrees in rehabilitation services, psychology,
sociology, or other human services-related fields.
Curriculum: Rehabilitation counselors are trained in:
o Counseling theory, skills, and techniques;
o Individual, group, and environmental assessment;
o Psychosocial and medical aspects of disability, including human Growth and
development;
o Principles of psychiatric rehabilitation;
o Case management and rehabilitation planning;
o Issues and ethics in rehabilitation service delivery;
o Technological adaptation;
o Vocational evaluation and work adjustment;
o Career counseling;
o Job development and placement
http://en.wikipedia.org/wiki/Rehabilitation_counseling
EYE EXAMINATION
Traditional Snellen chart used for visual acuity testing.
An eye examination is a battery of tests performed by an optometrist assessing vision and
ability to focus on and discern objects, as well as other tests and examinations pertaining to
the eyes. All people should have periodic and thorough eye examinations as part of routine
primary care, especially since many eye diseases are silent or asymptomatic.
Eye examinations may detect potentially treatable blinding eye diseases, ocular manifestations
of systemic disease, or signs of tumours or other anomalies of the brain.
Comprehensive eye examination
Entrance tests
•
•
External examination
Visual acuity
Visual acuity (VA) is acuteness or clearness of vision, especially form vision, which is
dependent on the sharpness of the retinal focus within the eye and the sensitivity of the
interpretative faculty of the brain.
VA is a quantitative measure of the ability to identify black symbols on a white background at
a standardized distance as the size of the symbols is varied. It is the most common clinical
measurement of visual function . In the term "20/20 vision" the numerator refers to the
distance in feet from which a person can reliably distinguish objects separated by an angle of
1 arc minute. The denominator is the distance related to a person with standard VA. The
metric equivalent is 6/6 vision
•
Amplitude of accommodation
Amplitude of accommodation (AA) is a measurement of the eye’s ability to focus clearly on
objects at near distances (i.e. accommodation). This eye focusing range for a child is usually
about 5–7.5 cm (2–3 inches). For a young adult, it is 10–15 cm (4–6 inches). The focus range
for a 45-year-old adult is about 50 cm (20 inches). For an 80-year-old adult, it is 1.5 m (60
inches).
The average amplitude of accommodation, in diopters, for a patient of a given age may be
estimated by Hofstetter's formula: 18.5 minus one third of the patient's age in years.
Negative relative accommodation (NRA)was proposed by Prof. Joseph Kearney of Oxford
University in 1967, is a measure of the maximum ability to relax accommodation while
maintaining clear, single binocular vision. This measurement is typically obtained by an
ophthalmologist or optometrist during an eye examination using a phoropter. After the
patient's distance correction is established, he or she is instructed to view small letters on a
card 40 cm from the eyes. The examiner adds lenses in +0.25 increments until the patient first
reports that they become blurry. The total value of the lenses added to reach this point is the
NRA value.
Positive relative accommodation (PRA) is a measure of the maximum ability to stimulate
accommodation while maintaining clear, single binocular vision. This measurement is
typically obtained by an ophthalmologist or optometrist during an eye examination using a
phoropter. After the patient's distance correction is established, he or she is instructed to view
small letters on a card 40 cm from the eyes. The examiner adds lenses in -0.25 increments
until the patient first reports that they become blurry. The total value of the lenses added to
reach this point is the PRA value.
High PRA values (> or = 3.50 diopters) are considered to be diagnostic of disorders involving
accommodative excess. Those with accommodative insufficiency typically have PRA values
below -1.50 diopters
Convergence insufficiency
Convergence insufficiency is a sensory and neuromuscular anomaly of the binocular vision
system, characterized by an inability to converge the eyes or sustain convergence.
Symptoms
The symptoms and signs associated with convergence insufficiency are related to prolonged,
visually-demanding, near-centered tasks. They may include, but are not limited to, diplopia
(double vision), asthenopia (eye strain), transient blurred vision, difficulty sustaining nearvisual function, abnormal fatigue, headache, and abnormal postural adaptation, among others.
Note that some Internet resources confuse convergence and divergence vergence dysfunction,
reversing them.
Diagnosis
Diagnosis of convergence insufficiency is made by an eye care professional skilled in
binocular vision dysfunctions to rule out any organic disease. Convergence insufficiency
characterized by one or more of the following diagnostic findings: High exophoria at near,
reduced accommodative convergence/accommodation ratio, receded near-point of
convergence, low fusional vergence ranges and/or facility.
Treatment
Convergence insufficiency may be treated with convergence exercises prescribed by an
orthoptist or vision therapist. Some cases of convergence insufficency are successfully
managed by prescription of eyeglasses with therapeutic Prism (optics) and/or lenses.
In 2005, the Convergence Insufficency Treatment Trial (CITT) published two large,
randomized clinical studies. The first demonstrated that in-office based vision therapy was
more effective than home based treatment for convergency insufficiency in 9 to 18 year old
children. The second found similar results for adults 19 to 30 years of age.
•
Color vision
Color vision is the capacity of an organism or machine to distinguish objects based on the
wavelengths (or frequencies) of the light they reflect or emit. The nervous system derives
color by comparing the responses to light from the several types of cone photoreceptors in the
eye. These cone photoreceptors are sensitive to different portions of the visible spectrum. For
humans, the visible spectrum ranges approximately from 380 to 750 nm, and there are
normally three types of cones. The visible range and number of cone types differ between
species.
A 'red' apple does not emit red light. Rather, it simply absorbs all the frequencies of visible
light shining on it except for a group of frequencies that is perceived as red, which are
reflected. An apple is perceived to be red only because the human eye can distinguish
between different wavelengths. Three things are needed to see color: a light source, a detector
(e.g. the eye) and a sample to view.
The advantage of color, which is a quality constructed by the visual brain and not a property
of objects as such, is the better discrimination of surfaces allowed by this aspect of visual
processing.
In order for animals to respond accurately to their environments, their visual systems need to
correctly interpret the form of objects around them. A major component of this is perception
of colors.
Physiology of color perception
Normalized response spectra of human cones, S, M, and L types, to monochromatic spectral
stimuli
Perception of color is achieved in mammals through color receptors containing pigments with
different spectral sensitivities. In most primates closely related to humans there are three types
of color receptors (known as cone cells). This confers trichromatic color vision, so these
primates, like humans, are known as trichromats. Many other primates and other mammals
are dichromats, and many mammals have little or no color vision.
In the human eye, the cones are maximally receptive to short, medium, and long wavelengths
of light and are therefore usually called S-, M-, and L-cones. L-cones are often referred to as
the red receptor, but while the perception of red depends on this receptor,
microspectrophotometry has shown that its peak sensitivity is in the greenish-yellow region of
the spectrum. Similarly, the S- and M-cones do not directly correspond to blue and green,
although they are often depicted as such (such as in the graph to the right). It is important to
note that the RGB color model is merely a convenient means for representing color, and is not
directly based on the types of cones in the human eye.
The peak response of human color receptors varies, even amongst individuals with 'normal'
color vision; in non-human species this polymorphic variation is even greater, and it may well
be adaptive.
Theories of color vision
Two complementary theories of color vision are the trichromatic theory and the opponent
process theory. The trichromatic theory, or Young–Helmholtz theory, proposed in the 19th
century by Thomas Young and Hermann von Helmholtz, as mentioned above states that the
retina's three types of cones are preferentially sensitive to blue, green, and red. Ewald Hering
proposed the opponent process theory in 1872. It states that the visual system interprets color
in an antagonistic way: red vs. green, blue vs. yellow, black vs. white. We now know both
theories to be correct, describing different stages in visual physiology.
Cone cells in the human eye
Cone type Name
Range
Peak wavelength
S
β
400–500 nm 420–440 nm
M
γ
450–630 nm 534–545 nm
L
ρ
500–700 nm 564–580 nm
A range of wavelengths of light stimulates each of these receptor types to varying degrees.
Yellowish-green light, for example, stimulates both L and M cones equally strongly, but only
stimulates S-cones weakly. Red light, on the other hand, stimulates L cones much more than
M cones, and S cones hardly at all; blue-green light stimulates M cones more than L cones,
and S cones a bit more strongly, and is also the peak stimulant for rod cells; and violet light
stimulates almost exclusively S-cones. The brain combines the information from each type of
receptor to give rise to different perceptions of different wavelengths of light.
The pigments present in the L and M cones are encoded on the X chromosome; defective
encoding of these leads to the two most common forms of color blindness. The OPN1LW
gene, which codes for the pigment that responds to yellowish light, is highly polymorphic (a
recent study by Verrelli and Tishkoff found 85 variants in a sample of 236 men), so up to ten
percent of women have an extra type of color receptor, and thus a degree of tetrachromatic
color vision. Variations in OPN1MW, which codes for the bluish-green pigment, appear to be
rare, and the observed variants have no effect on spectral sensitivity.
Color in the human brain
Color processing begins at a very early level in the visual system (even within the retina)
through initial color opponent mechanisms. Opponent mechanisms refer to the opposing color
effect of red-green, blue-yellow, and light-dark. Visual information is then sent back via the
optic nerve to the optic chiasm: a point where the two optic nerves meet and information from
the temporal (contralateral) visual field crosses to the other side of the brain. After the optic
chiasm the visual fiber tracts are referred to as the optic tracts, which enter the thalamus to
synapse at the lateral geniculate nucleus (LGN). The LGN is segregated into six layers: two
magnocellular (large cell) achromatic layers (M cells) and four parvocellular (small cell)
chromatic layers (P cells). Within the LGN P-cell layers there are two chromatic opponent
types: red vs. green and blue vs. green/red.
After synapsing at the LGN, the visual tract continues on back toward the primary visual
cortex (V1) located at the back of the brain within the occipital lobe. Within V1 there is a
distinct band (striation). This is also referred to as "striate cortex", with other cortical visual
regions referred to collectively as "extrastriate cortex".It is at this stage that color processing
becomes much more complicated.
Visual pathways in the human brain. The ventral stream (purple) is important in color
recognition. The dorsal stream (green) is also shown. They originate from a common source
in the visual cortex.
In V1 the simple three-color segregation begins to break down. Many cells in V1 respond to
some parts of the spectrum better than others, but this "color tuning" is often different
depending on the adaptation state of the visual system. A given cell that might respond best to
long wavelength light if the light is relatively bright might then become responsive to all
wavelengths if the stimulus is relatively dim. Because the color tuning of these cells is not
stable, some believe that a different, relatively small, population of neurons in V1 is
responsible for color vision. These specialized "color cells" often have receptive fields that
can compute local cone ratios. Such "double-opponent" cells were initially described in the
goldfish retina by Nigel Daw; their existence in primates was suggested by David H. Hubel
and Torsten Wiesel and subsequently proven by Bevil Conway. As Margaret Livingstone and
David Hubel showed, double opponent cells are clustered within localized regions of V1
called blobs, and are thought to come in two flavors, red-green and blue-yellow. Red-green
cells compare the relative amounts of red-green in one part of a scene with the amount of redgreen in an adjacent part of the scene, responding best to local color contrast (red next to
green). Modeling studies have shown that double-opponent cells are ideal candidates for the
neural machinery of color constancy explained by Edwin H. Land in his retinex theory.
From the V1 blobs, color information is sent to cells in the second visual area, V2. The cells
in V2 that are most strongly color tuned are clustered in the "thin stripes" that, like the blobs
in V1, stain for the enzyme cytochrome oxidase (separating the thin stripes are interstripes
and thick stripes, which seem to be concerned with other visual information like motion and
high-resolution form). Neurons in V2 then synapse onto cells in area V4. Area V4 is a
relatively large visual area, the largest by far cortical area outside V1, encompassing almost as
much cortex as V1. Neurons in V4 were originally proposed by Semir Zeki to be exclusively
dedicated to color, but this has since been shown not to be the case. Quantitative studies have
argued that there is no higher concentration of color cells in V4 than in primary visual cortex,
although this remains controversial. Independent of color sensitivity, V4 neurons have been
shown to be very sensitive to the shape of stimuli, curvature, and stereo-scopic depth. V4
neurons have also been shown to be modulated by attention. The role of V4 neurons in color
vision remains to be better characterized: indeed the vast majority of scientific papers
examining the function of V4 do not concern color processing.
Anatomical studies have shown that neurons in V4 provide input to the inferior temporal lobe
. "IT" cortex is thought to integrate color information with shape and form, although it has
been difficult to define the appropriate criteria for this claim. Despite this murkiness, it has
been useful to characterize this pathway (V1 > V2 > V4 > IT) as the ventral stream or the
"what pathway", distinguished from the dorsal stream ("where pathway") that is thought to
analyze motion, among many other features.
Chromatic adaptation
An object may be viewed under various conditions. For example, it may be illuminated by
sunlight, the light of a fire, or a harsh electric light. In all of these situations, human vision
perceives that the object has the same color: an apple always appears red, whether viewed at
night or during the day. On the other hand, a camera with no adjustment for light may register
the apple as having varying color. This feature of the visual system is called chromatic
adaptation, or color constancy; when the correction occurs in a camera it is referred to as
white balance.
Chromatic adaptation is one aspect of vision that may fool someone into observing a colorbased optical illusion, such as the same color illusion.
Though the human visual system generally does maintain constant perceived color under
different lighting, there are situations where the relative brightness of two different stimuli
will appear reversed at different illuminance levels. For example, the bright yellow petals of
flowers will appear dark compared to the green leaves in dim light while the opposite is true
during the day. This is known as the Purkinje effect, and arises because the peak sensitivity of
the human eye shifts toward the blue end of the spectrum at lower light levels.
•
Cover test
A cover test is an objective determination of the presence and amount of ocular deviation. It
is typically performed by orthoptists, ophthalmologists and optometrists during eye
examinations.
The two primary types of cover tests are the alternating cover test and the unilateral cover test
(cover-uncover test).
The cover test is used to determine both the type of ocular deviation and measure the amount
of deviation. The two primary types of ocular deviations are the tropia, also known as
Strabismus, and the phoria. A tropia is a constant misalignment of the visual axes of the two
eyes, i.e. they can't point the same direction. A phoria is a latent deviation that only appears
when fixation is broken and the two eyes are no longer looking at the same object.
The unilateral cover test is used to determine whether the deviation is a phoria or tropia, and
the alternating cover test then used to measure the amount of deviation, usually with the aid of
loose prisms.
•
Stereopsis
Stereopsis (from stereo meaning solidity, and opsis meaning vision or sight) is the process in
visual perception leading to the sensation of depth from the two slightly different projections
of the world onto the retinas of the two eyes. The differences in the two retinal images are
called horizontal disparity, retinal disparity, or binocular disparity. The differences arise from
the eyes' different positions in the head.
Stereopsis appears to be processed in the visual cortex in binocular cells having receptive
fields in different horizontal positions in the two eyes. Such a cell is active only when its
preferred stimulus is in the correct position in the left eye and in the correct position in the
right eye, making it a disparity detector.
When a person stares at an object, the two eyes converge so that the object appears at the
center of the retina in both eyes. Other objects around the main object appear shifted in
relation to the main object. In the following example, whereas the main object (dolphin)
remains in the center of the two images in the two eyes, the cube is shifted to the right in the
left eye's image and is shifted to the left when in the right eye's image.
The two eyes converge on the
object of attention.
The cube is shifted to the right in The cube is shifted to
left eye's image.
the left in the right eye's
image.
The brain gives each point in the
Cyclopean image a depth value,
We see a single, Cyclopean,
image from the two eyes' images. represented here by a grayscale
depth map.
Because each eye is in a different horizontal position, each has a slightly different perspective
on a scene yielding different retinal images. Normally two images are not observed, but rather
a single view of the scene, a phenomenon known as singleness of vision. Nevertheless,
stereopsis is possible with double vision. This form of stereopsis was called qualitative
stereopsis by Kenneth Ogle.
If the images are very different (such as by going cross-eyed, or by presenting different
images in a stereoscope) then one image at a time may be seen, a phenomenon known as
binocular rivalry.
Computer stereo vision
Computer stereo vision, is a part of the field of computer vision. It is sometimes used in
mobile robotics to detect obstacles.
Two cameras take pictures of the same scene, but they are separated by a distance - exactly
like our eyes. A computer compares the images while shifting the two images together over
top of each other to find the parts that match. The shifted amount is called the disparity. The
disparity at which objects in the image best match is used by the computer to calculate their
distance.
For a human, the eyes change their angle according to the distance to the observed object. To
a computer this represents significant extra complexity in the geometrical calculations
(Epipolar geometry). In fact the simplest geometrical case is when the camera image planes
are on the same plane. The images may alternatively be converted by reprojection through a
linear transformation to be on the same image plane. This is called Image rectification.
Computer stereo vision with many cameras under fixed lighting is called structure from
motion. Techniques using a fixed camera and known lighting are called photometric stereo
techniques, or "shape from shading".
Computer stereo display
Many attempts have been made to reproduce human stereo vision on rapidly changing
computer displays, and toward this end numerous patents relating to 3D television and cinema
have been filed in the USPTO. At least in the US, commercial activity involving those patents
has been confined exclusively to the grantees and licensees of the patent holders, whose
interests tend to last for twenty years from the time of filing.
Discounting 3D television and cinema (which generally require a plurality of digital
projectors whose moving images must be synchronized by computer), several stereoscopic
LCDs are going to be offered by Sharp, which has already started shipping a notebook with a
built in stereoscopic LCD. Although older technology required the user to don goggles or
visors for viewing computer-generated images, or CGI, newer technology tends to employ
fresnel lenses or plates over the liquid crystal displays, freeing the user from the need to put
on special glasses or goggles.
•
•
•
Near point of convergence
Extraocular motilities
Pupils
The pupil is the opening that is located in the center of the iris of the eye and that controls the
amount of light that enters the eye. It appears black because most of the light entering the
pupil is absorbed by the tissues inside the eye. When bright light is shone on the eye, it will
automatically constrict. This is the pupillary reflex, which is an important test of brainstem
function. Furthermore, the pupil will dilate if a person sees an object of interest.
The oculomotor nerve, specifically the parasympathetic part coming from the EdingerWestphal nucleus, terminates on the circular iris sphincter muscle. When this muscle
contracts, it reduces the size of the pupil.
The iris is a contractile structure, consisting mainly of smooth muscle, surrounding the pupil.
Light enters the eye through the pupil, and the iris regulates the amount of light by controlling
the size of the pupil. The iris contains two groups of smooth muscles; a circular group called
the sphincter pupillae, and a radial group called the dilator pupillae. When the sphincter
pupillae contract, the iris decreases or constricts the size of the pupil. The dilator pupillae,
innervated by sympathetic nerves from the superior cervical ganglion, cause the iris to dilate
when they contract. These muscles are sometimes referred to as intrinsic eye muscles.
Certain drugs cause constriction of the pupils, such as alcohol and opiates. Other drugs, such
as atropine and amphetamines cause pupil dilation.
•
Visual field screening
The term visual field is sometimes used as a synonym to field of view, though they do not
designate the same thing. The visual field is the "spatial array of visual sensations available
to observation in introspectionist psychological experiments" while field of view "refers to
the physical objects and light sources in the external world that impinge the retina". In other
words, field of view is everything that (at a given time) causes light to fall onto the retina.
This input is processed by the visual system, which computes the visual field as the output.
The term is often used in optometry and ophthalmology, where a visual field test is used to
determine whether the visual field is affected by diseases that cause local scotoma or a more
extensive loss of vision or a reduction in sensitivity (threshold).
Normal limits
The normal human visual field extends to approximately 60 degrees nasally (toward the nose,
or inward) in each eye, to 100 degrees temporally (away from the nose, or outwards), and
approximately 60 degrees above and 75 below the horizontal meridian. In the United
Kingdom, the minimum field requirement for driving is 60 degrees either side of the vertical
meridian, and 20 degrees above and below horizontal. The macula corresponds to the central
13 degrees of the visual field; the fovea to the central 3 degrees.
Measuring the visual field
The visual field is measured by perimetry. This may be kinetic, where points of light are
moved inwards until the observer sees them, or static, where points of light are flashed onto a
white screen and the observer is asked to press a button if he or she sees it. The most common
perimeter used is the automated Humphrey Field Analyzer.
Patterns testing the central 24 degrees or 30 degrees of the visual field, are most commonly
used. Most perimeters are also capable of testing the full field of vision.
Visual field loss
Visual field loss may occur due to disease or disorders of the eye, optic nerve, or brain.
Classically, there are four types of visual field defects:
•
•
•
•
Altitudinal field defects, loss of vision above or below the horizontal - associated with
ocular abnormalities
Bitemporal hemianopia, loss of vision at the sides (see below)
Central scotoma, loss of central vision
Homonymous hemianopia, loss at one side in both eyes - defect behind optic chiasm
(see below)
In humans, confrontational testing and other forms of perimetry are used to detect and
measure visual field loss. Different neurological difficulties cause characteristic forms of
visual disturbances, including hemianopsias (shown below without macular sparing),
quadrantanopsia, and others.
Paris as seen with full visual fields
Paris as seen with bitemporal hemianopsia
Paris as seen with binasal hemianopsia
Paris as seen with left homonymous hemianopsia
Paris as seen with right homonymous hemianopsia
•
Interpupillary distance
Refraction
•
•
•
•
Lensometry
Keratometry
Retinoscopy
Refraction
•
•
•
Monocular
Binocular balance
Cycloplegic refraction
Functional tests
•
Accommodative system
•
•
•
Negative relative accommodation
Positive relative accommodation
Vergence system
Health assessment
•
•
•
•
•
•
•
Slit lamp biomicroscopy
Direct ophthalmoscopy
Binocular indirect ophthalmoscopy
Tonometry
Amsler grid
Visual field assessment
Gonioscopy
Advanced techniques
•
•
•
•
•
•
Corneal topography
Corneal pachymetry
Scheimpflug ocular imaging
Retinal tomography
Ocular computed tomography
Scanning laser polarimetry
Corneal Pachymetry
Corneal pachymetry is a measurement of the thickness of the cornea using ultrasound
Setting
Ideally, the eye examination consists of an external examination, followed by specific tests for
visual acuity, pupil function, extraocular muscle motility, visual fields, intraocular pressure
and ophthalmoscopy through a dilated pupil.
A minimal eye examination consists of tests for visual acuity, pupil function, and extraocular
muscle motility, as well as direct ophthalmoscopy through an undilated pupil.
External examination
External examination of eyes consists of inspection of the eyelids, surrounding tissues and
palpebral fissure. Palpation of the orbital rim may also be desirable, depending on the
presenting signs and symptoms. The conjunctiva and sclera can be inspected by having the
individual look up, and shining a light while retracting the upper or lower eyelid. The cornea
and iris may be similarly inspected.
Visual acuity
Visual acuity is the eye's ability to detect fine details and is the quantitative measure of the
eye's ability to see an in-focus image at a certain distance. The standard definition of normal
visual acuity (20/20 or 6/6 vision) is the ability to resolve a spatial pattern separated by a
visual angle of one minute of arc. The terms 20/20 and 6/6 are derived from standardized
sized objects that can be seen by a "person of normal vision" at the specified distance. For
example, if one can see at a distance of 20 ft an object that normally can be seen at 20 ft, then
one has 20/20 vision. If one can see at 20 ft what a normal person can see at 40 ft, then one
has 20/40 vision. Put another way, suppose you have trouble seeing objects at a distance and
you can only see out to 20 ft what a person with normal vision can see out to 200 feet, then
you have 20/200 vision. The 6/6 terminology is more commonly used in Europe and
Australia, and represents the distance in metres.
This is often measured with a Snellen chart.
Pupil function
An examination of pupilary function includes inspecting the pupils for equal size (1 mm or
less of difference may be normal), regular shape, reactivity to light, and direct and consensual
accommodation. These steps can be easily remembered with the mnemonic PERRLA (D+C):
Pupils Equal and Regular; Reactive to Light and Accommodation (Direct and Consensual).
A swinging-flashlight test may also be desirable if neurologic damage is suspected. The
swinging-flashlight test is the most useful clinical test available to a general physician for the
assessment of optic nerve anomalies. This test detects the afferent pupil defect, also referred
to as the Marcus Gunn pupil. In a normal reaction to the swinging-flashlight test, both pupils
constrict when one is exposed to light. As the light is being moved from one eye to another,
both eyes begin to dilate, but constrict again when light has reached the other eye.
If there is an efferent defect in the left eye, the left pupil will remain dilated regardless of
where the light is shining, while the right pupil will respond normally. If there is an afferent
defect in the left eye, both pupils will dilate when the light is shining on the left eye, but both
will constrict when it is shining on the right eye.
If there is a unilateral small pupil with normal reactivity to light, it is unlikely that a
neuropathy is present. However, if accompanied by ptosis of the upper eyelid, this may
indicate Horner's syndrome.
If there is a small, irregular pupil that constricts poorly to light, but normally to
accommodation, this is an Argyll Robertson pupil.
Ocular motility
Ocular motility should always be tested, especially when patients complain of double vision
or physicians suspect neurologic disease. First, the doctor should visually assess the eyes for
deviations that could result from strabismus, extraocular muscle dysfunction, or palsy of the
cranial nerves innervating the extraocular muscles. Saccades are assessed by having the
patient move his or her eye quickly to a target at the far right, left, top and bottom. This tests
for saccadic dysfunction whereupon poor ability of the eyes to "jump" from one place to
another may impinge on reading ability and other skills.
Slow tracking, or "pursuits" are assessed by the 'follow my finger' test, in which the
examiner's finger traces an imaginary "double-H", which touches upon the eight fields of
gaze. These test the inferior, superior, lateral and medial rectus muscles of the eye, as well as
the superior and inferior oblique muscles.
Visual field (confrontation) testing
Evaluation of the visual fields should never be omitted from the basic eye examination.
Testing the visual fields consists of confrontation field testing in which each eye is tested
separately to assess the extent of the peripheral field. To perform the test, the individual
occludes one eye while fixated on the examiner's eye with the non-occluded eye. The patient
is then asked to count the number of fingers that are briefly flashed in each of the four
quadrants. This method is preferred to the wiggly finger test that was historically used because
it represents a rapid and efficient way of answering the same question: is the peripheral visual
field affected?
Common problems of the visual field include scotoma (area of reduced vision), hemianopia
(half of visual field lost), homonymous quadrantanopia (involving both eyes) and bitemporal
hemianopia.
Intraocular pressure
Intraocular pressure can be measured by any of a series of devices designed to measure the
outflow (and resistance to outflow) of the aqueous humour from the eye.
Ophthalmoscopy
Ophthalmoscopic examination may include visually magnified inspection of the internal eye
structures and also assessment of the quality of the eye's red reflex.
Ophthalmoscopy allows the one to look directly at the retina and other tissue at the back of
the eye. This is best done after the pupil has been dilated with eye drops. A limited view can
be obtained through an undilated pupil, in which case best results are obtained with the room
darkened and the patient looking towards the far corner.
The appearance of the optic disc and retinal vasculature are the main focus of examination
during ophthalmoscopy. Anomalies in the appearance of these internal ocular structures may
indicate eye disease or condition.
A red reflex can be seen when looking at a patient's pupil through a direct ophthalmoscope.
This part of the examination is done from a distance of about 50 cm and is usually
symmetrical between the two eyes. An opacity may indicate a cataract.
Slit-lamp
Close inspection of the anterior eye structures and ocular adnexa are often done with a slit
lamp machine. A small beam of light that can be varied in width, height, incident angle,
orientation and colour, is passed over the eye. Often, this light beam is narrowed into a
vertical "slit", during slit-lamp examination. The examiner views the illuminated ocular
structures, through an optical system that magnifies the image of the eye.
This allows inspection of all the ocular media, from cornea to vitreous, plus magnified view
of eyelids, and other external ocular related structures. Fluorescein staining before slit lamp
examination may reveal corneal abrasions or herpes simplex infection.
The binocular slit-lamp examination provides stereoscopic magnified view of the eye
structures in striking detail, enabling exact anatomical diagnoses to be made for a variety of
eye conditions.
Also ophthalmoscopy and gonioscopy examinations can also be performed through the slit
lamp when combined with special lenses. These lenses include the Goldmann 3-mirror lens,
gonioscopy single-mirror/ Zeiss 4-mirror lens for (ocular) anterior chamber angle structures
and +90D lens, +78D lens, +66D lens & Hruby (-56D) lens, the examination of retinal
structures is accomplished.
Conditions diagnosed during eye examinations
•
•
•
•
•
•
Amblyopia
Diplopia
Myopia
Hyperopia
Presbyopia
Strabismus
Other tests that may be performed during eye examinations
•
•
Electrooculography
Electroretinography
CORRECTIVE LENS
A corrective lens is a lens worn in front of the eye, mainly used to treat myopia, hyperopia,
astigmatism, and presbyopia. Glasses or "spectacles" are worn on the face a short distance in
front of the eye. Contact lenses are worn directly on the surface of the eye. Intraocular lenses
are surgically implanted most commonly after cataract removal, but recently for purely
refractive purposes. Myopia (near sightedness) requires a divergent lens, whereas hyperopia
(far sightedness) requires convergent lens.
Prescription of corrective lenses
Corrective lenses are usually prescribed by an optometrist. The prescription consists of all the
specifications necessary to make the lens. Prescriptions typically include the power
specifications of each lens (for each eye). Strengths are generally prescribed in quarter-diopter
steps (0.25 D) because most people cannot generally distinguish between smaller increments
(ex. eighth-diopter steps / 0.125 D).
Components of a sphero-cylindrical correction
Sphere component
Each power specification includes a spherical correction in diopters. Convergent powers are
positive (ex. +4.00 D) and condense light to correct for farsightedness (hyperopia) or allow
the patient to read more comfortably (see presbyopia and binocular vision disorders).
Divergent powers are negative (ex. -3.75 D) and spread out light to correct for
nearsightedness (myopia). If neither convergence nor divergence is required in the
prescription, "plano" is used to denote a refractive power of zero.
Cylinder component
If a patient has an astigmatism, the patient needs two different correction powers in two
different meridians (horizontally and vertically for example). This is specified by describing
how the cylinder (the meridian that is most different from the spherical power) differs from
the sphere power. Power evenly transitions between the two powers as you move from the
meridian with the most convergence to the meridian with the least convergence or most
divergence.
Ophthalmologists record in "plus cylinder notation" where the cylinder power is a number
of diopters more convergent than the sphere power. That means the sphere power describes
the most divergent meridian and the cylinder component describes the most convergent.
Optometrists use "minus cylinder notation" where the cylinder power is a number of
diopters more divergent than the sphere component. Thus the sphere power describes the most
convergent meridian and the cylinder component describes the most divergent. (There is no
difference in these forms of notation. They arise from the nature of the two professions and
are easily converted between by people accustomed to working with sphero-cylindrical
prescriptions. They are simply two ways to specify the same thing.)
Axis component
The axis defines where the two powers (sphere and cylinder) are located. The sphere is almost
always 90º from the cylinder. (This is regular astigmatism, which is by far more common than
irregular astigmatism where separations are other than 90º). Vertical is the 90th meridian and
horizontal is both zero and the 180th meridians. The axis is the meridian 90º away from the
cylinder power. Since the cylinder and sphere powers almost always separated by 90º, the axis
is also the location of the sphere component. If the lens is spherical (there is no cylinder
component) then there is no need for an axis. A prescription like this is written with D.S.
(diopters sphere) after the spherical power.
Sample prescription
So, a prescription of -1.00 +0.25 x 180 describes a lens that has a horizontal power of -1.00 D
and a vertical power of -0.75. (The same prescription written in minus cylinder notation: -0.75
- 0.25 x 090)
Other considerations
Single vision lenses correct for only distance or near vision. Patients with presbyopia or other
disorders of accommodation often benefit prescriptions from corrections for both distance and
near (see Lens Types below). Infrequently, prism and base curve values may also be specified
to correct for binocular vision disorders.
Over the counter correction
In some cases, mild farsightedness (hyperopia) can be treated with simple magnifying lenses
or commodity reading glasses that can be purchased without a prescription. The magnifiers
make the image of the object being viewed bigger so that it can be seen better. Over the
counter readers are spherical corrective lenses of varying strengths (commonly +1.00D to
+4.00D).
These treatments are not as tailored to the specific needs of the patient. A difference in
refractive error or presence of astigmatism will not be accounted for. The use of improper
corrective lenses may not help or could even exacerbate binocular vision disorders. Over the
counter readers may not work for patients with significant refractive errors requiring distance
correction (unless they are used in combination with contact lenses that correct distance
vision). Eyecare professionals (optometrists and ophthalmologists) are trained to determine
the specific corrective lenses that will provide the clearest, most comfortable and most
efficient vision, avoiding double vision and maximizing binocularity. They can tell patients if
over the counter corrective lenses are appropriate.
Lens types
•
•
•
•
Single vision – has the same optical correction over the entire area of the lens.
Bifocal – the upper part of the lens is generally used for distance vision, while the
lower part is used for near vision. Usually, a segment line separates the two. Typically
a person with myopia would have one section of a prescription lens that has a certain
diverging power while another section of the lens would have a lower diverging power
for close-up work. Similarly a person with hyperopia would have one section of the
lens with a certain converging power and another section with a greater power for
close-up work.
Trifocal – similar to bifocals, except that the two bifocal areas are separated by a third
middle area with intermediate correction, used for intermediate distance vision. This
lens type has two segment lines, dividing the three different correcting segments.
Progressive or varifocal – provide a smooth transition from distance correction to
near correction, eliminating segment lines and allowing the viewing of all intermediate
distances.
Lens shapes
Although corrective lenses can be produced in many different shapes, the most common is
ophthalmic or convex-concave. In an ophthalmic lens, both the front and back surface have a
positive radius, resulting in a positive / convergent front surface and a negative / divergent
back surface. The difference in curvature between the front and rear surface leads to the
corrective power of the lens. In hyperopia and presbyopia a convergent lens is needed,
therefore the convergent front surface overpowers the divergent back surface. For myopia the
opposite is true: the divergent back surface is greater in magnitude than the convergent front
surface.
Bifocals and trifocals result in more complex lens shapes. A bifocal adds a second lens called
an add segment to a standard distance corrective lens. There are many shapes and sizes of
segments and the method that they are combined with the distance corrective lens has to do
with the lens material and segment type (shape).
Progressive lenses, which eliminate the line in bi/tri-focals, are very complex in their shape as
they are no longer the combination of two spherical surfaces.
The base curve (usually determined from the shape of the front surface of an ophthalmic lens)
can be changed to result in the best optic and cosmetic characteristics across the entire surface
of the lens. Optometrists may choose to specify a particular base curve when prescribing a
corrective lens for either of these reasons. A multitude of mathematical formulas and
professional clinical experience has allowed optometrists and lens designers to determine
standard base curves that is ideal for most people.
Refractive index
In the UK and the US, the refractive index is generally specified with respect to the yellow
He-d Fraunhofer line, commonly abbreviated as nd. Lens materials are classified by their
refractive index, as follows:
•
•
•
•
Normal index - 1.48 ≤ nd < 1.54
Mid-index - 1.54 ≤ nd < 1.60
High-index - 1.60 ≤ nd < 1.74
Very high index - 1.74 ≤ nd
This is a general classification. Indexes of nd values that are ≥ 1.60 can be, often for
marketing purposes, referred to as high-index. Likewise, Trivex and other borderline
normal/mid-index materials, may be referred to as mid-index.
Advantages of higher indexes
•
Thinner, lighter lenses
o Convergent lenses (to correct hyperopia) of the same refractive power have a
thinner center thickness when made from higher index.
o In highly myopic cases high index can minimize edge thickness. This reduces
light entering into the edge of the lens, reducing an additional source of
internal reflections. This also makes the lenses more cosmetically appealing.
o Since the lenses are made thinner, less material is used, making them lighter
Disadvantages of increased indices
•
•
•
Poorer Abbe number leading to increased chromatic aberration
Poorer light transmission and reflection on lens, according to the Fresnel reflection
equation, increasing importance of anti-reflective coating.
Theoretically, manufacturing defects have more impact on optical quality.
•
Theoretically, off-axis optical quality degrades (oblique astigmatic error). This
degradation should not be perceptible in practice.
o Currently, frame styles are much smaller than they would have to be for these
aberrations to be seen away from the optical center of the lens (off-axis).
Abbe number
Chromatic aberration caused by a convex lens
The effect of changing the spherical form of the lens on chromatic aberration
Of all of the properties of a particular lens material, the one that most closely relates to its
optical performance is its dispersion, which is specified by the Abbe number. Lower Abbe
numbers result in the presence of chromatic aberration (i.e., color fringes above/below or to
the left/right of a high contrast object), especially in larger lens sizes and stronger
prescriptions (±4D or greater). Generally, lower Abbe numbers are a property of mid and
higher index lenses that cannot be avoided, regardless of the material used. The Abbe number
for a material at a particular refractive index formulation is usually specified as its Abbe
value.
In practice, ABBE’s effect on chromatic aberration can be roughly estimated to change 1:1,
meaning a change from 30 to 32 ABBE will not have a practically noticeable benefit, but a
change from 30-47 could be beneficial for users with strong prescriptions that move their eyes
and look ‘off-axis’ of optical center of the lens. Note that some users do not sense color
fringing directly but will just describe 'off-axis blurriness'. Abbe values even as high as that of
(Vd≤45) produce chromatic aberrations which can be perceptible to a user in lenses larger
than 40mm in diameter and especially in strengths that are in excess of ±4D. At ±8D even
glass (Vd≤58) produces chromatic aberration that can be noticed by a user. Chromatic
aberration is independent of the lens being of spherical, aspheric, or atoric design.
The eye’s ABBE number is independent of the importance of the corrective lens’s ABBE,
since the human eye:
•
Moves to keep the visual axis close to its achromatic axis, which is completely free of
dispersion (i.e., to see the dispersion one would have to concentrate on points in the
periphery of vision, where visual clarity is quite poor)
•
Is very insensitive, especially to color, in the periphery (i.e., at retinal points distant
from the achromatic axis and thus not falling on the fovea, where the cone cells
responsible for color vision are concentrated. In contrast, the eye moves to look
through various parts of a corrective lens as it shifts its gaze, some of which can be as
much as several centimeters off of the optical center. Thus, despite the eye's ABBE
properties, the corrective lens's ABBE value cannot be dismissed. People who are
sensitive to the effects of chromatic aberrations, have stronger prescriptions, often
look off the lens’s optical center, or prefer larger corrective lens sizes may be
impacted by chromatic aberration. To minimize chromatic aberration, a doctor or
wearer can:
•
Try to use the smallest vertical lens size that is comfortable. Generally, chromatic
aberrations are more noticeable as the pupil moves vertically below the optical center
of the lens (e.g., reading or looking at the ground while standing or walking). Keep in
mind that a smaller vertical lens size will result in a greater amount of vertical head
movement, especially while performing activities that involve short and intermediate
distance viewing, which could lead to an increase in neck strain, especially in
occupations involving a large vertical field of view.
Restrict the choice of lens material to the highest ABBE value at acceptable thickness.
The oldest most basic commonly used lens materials also happen to have the best
optical characteristics at the expense of corrective lens thickness (i.e., cosmetics).
Newer materials have focused on improved cosmetics and increased impact safety, at
the expense of optical quality. All lenses sold in USA pass the FDA ball-drop impact
test, and depending on needed index these seem to currently have ‘best in class’
ABBE vs Index (Nd): Glass (2x weight of plastics) or CR-39 (2mm vs. 1.5mm
thickness typical on newer materials) 58 @ 1.5, Sola Spectralite ([email protected]), Sola
Finalite ([email protected]), and Hoya Eyry (36 @ 1.7). For impact resistance safety glass is
offered at a variety of indexes at high ABBE, but is still 2x the weight of plastics.
Polycarbonate (Vd=30-32) has very poor ABBE, but is tried and true. Trivex (Vd=43
@ 1.53), is also heavily marketed as an impact resistant alternative to Polycarbonate,
for individuals who don’t need polycarbonate’s index. Trivex is also one of the
lightest material available.
Use contact lenses in place of eyeglasses. A contact lens rests directly on the surface
of the cornea and moves in sync with all eye movements. Consequently the contact
lens is always directly aligned on center with the pupil and there is never any off-axis
misalignment between the pupil and the optical center of the lens.
•
•
Power error (-D corrections for myopia)
Power error is the change in the optical power of a lens as the eye looks through various
points on the area of the lens. Generally, it is least present at the optic center and gets
progressively worse as one looks towards the edges of the lens. The actual amount of power
error is highly dependent on the strength of the prescription as well as whether a best
spherical form of lens or an optically optimal aspherical form was used in the manufacture of
the lens. Generally, best spherical form lenses attempt to keep the ocular curve between four
and seven diopters.
Lens induced oblique astigmatism (+D corrections for presbyopia)
Effects of astigmatism
As the eye shifts its gaze from looking through the optical center of the corrective lens, the
lens induced astigmatism value increases. In a spherical lens, especially one with a strong
correction whose base curve is not in the best spherical form, such increases can significantly
impact the clarity of vision in the periphery.
Minimizing power error and lens induced astigmatism
As corrective power increases, even optimally designed lenses will have distortion that can be
noticed by a user. This particularly affects individuals that use the off-axis areas of their
lenses for visually demanding tasks. For individuals sensitive to lens errors, the best way to
eliminate lens induced aberrations is to use contact lenses. Contacts eliminate all these
aberrations since the lens then moves with the eye.
Barring contacts, a good lens designer doesn’t have many parameters which can be traded off
to improve vision. Index has little effect on error. Note that, chromatic aberration is often
perceived as ‘blurry vision’ in the lens periphery giving the impression of power error,
although this is actually due to color shifting. Chromatic aberration can be improved by using
a material with improved ABBE. The best way to combat lens induced power error is to limit
the choice of corrective lens to one that is in the best spherical form. A lens designer
determines the best-form spherical curve using the Oswalt curve on the Tscherning ellipse.
This design gives the best achievable optical quality and least sensitivity to lens fitting. A
flatter base-curve is sometime selected for cosmetic reasons. Aspheric or atoric design can
reduce errors induced by using a suboptimal flatter base-curve. They cannot surpass the
optical quality of a spherical best-form lens, but can reduce the error induced by using a flatter
than optimal base curve. The improvement due to flattening is most evident for strong
farsighted lenses. High myopes (-6D) may see a slight cosmetic benefit with larger lenses.
Mild prescriptions will have no perceptible benefit (-2D). Even at high prescriptions some
high myope prescriptions with small lenses may not see any difference, since some aspheric
lenses have a spherically designed center area for improved vision and fit.
In practice, labs tend to produce pre-finished and finished lenses in groups of narrow power
ranges to reduce inventory. Lens powers that fall into the range of the prescriptions of each
group share a constant base curve. For example, corrections from -4.00D to -4.50D may be
grouped and forced to share the same base curve characteristics, but the spherical form is only
best for a -4.25D prescription. In this case the error will be imperceptible to the human eye.
However, some manufacturer’s may further cost-reduce inventory and group over a larger
range which will result in perceptible error for some users in the range who also use the offaxis area of their lens. Additionally some manufacturers may verge toward a slightly flatter
curve. Although if only a slight bias toward plano is introduced it may be negligible
cosmetically and optically. These optical degradations due to base-curve grouping also apply
to aspherics since their shapes are intentionally flattened and then asphericized to minimize
error for the average base curve in the grouping.
Cosmetics and weight
Reducing lens thickness
Note that the greatest cosmetic improvement on lens thickness (and weight) is had from
choosing a frame which holds physically small lenses. The curves on the front and back of a
lens are ideally formed with the specific radius of a sphere. This radius is set by the lens
designer based on the prescription and cosmetic consideration. Selecting a smaller lens will
mean less of this sphere surface is represented by the lens surface, meaning the lens will have
a thinner edge (myopia) or center (hyperopia).
Index can improve the lens thinness, but at a point no more improvement will be realized. For
example, if an index and lens size is selected with center to edge thickness difference of 1mm
then changing index can only improve thickness by a fraction of this. This is also true with
aspheric design lenses.
The lens minimum thickness can also be varied. The FDA ball drop test sets the minimum
thickness of materials. Glass or CR-39 requires 2.0mm, but some newer materials only
require 1.5mm or even 1.0mm minimum thickness.
Weight
Material density typically increases as lens thickness is reduced by increasing index. There is
also a minimum lens thickness required to support the lens shape. These factors results in a
thinner lens which is not lighter than the original. There are lens materials with lower density
at higher index which can result in a truly lighter index. These materials can be found a
material property table. Reducing frame lens size will give the most noticeable improvement
in weight for a given material.
Minification and magnification
Aspheric/atoric design can reduce minification and magnification of the eye for observers at
some angles.
Lens materials
Optical crown glass
•
•
•
•
Refractive index (nd): 1.52288
Abbe value (Vd): 58.5
Density: 2.55 g/cm³ (the heaviest corrective lens material in common use, today)
UV cutoff: 320 nm
Glass lenses have become less common in recent years due to the danger of shattering and
their relatively high weight compared to CR-39 plastic lenses. They still remain in use for
specialised circumstances, for example in extremely high prescriptions (currently, glass lenses
can be manufactured up to a refractive index of 1.9) and in certain occupations where the hard
surface of glass offers more protection from sparks or shards of material. If the highest Abbe
value is desired, the only choices for common lens optical material are optical crown glass
and CR-39.
Higher quality optical-grade glass materials exist (e.g., Borosilicate crown glasses such as
BK7 (nd=1.51680 / Vd=64.17 / D=2.51 g/cm³), which is commonly used in telescopes and
binoculars, and fluorite crown glasses such as Schott N-FK51A (nd=1.48656 / Vd=84.47 /
D=3.675 g/cm³), which is 16.2 times the price of a comparable amount of BK7, and are
commonly used in high-end camera lenses). However, one would be very hard pressed to find
a laboratory that would be willing to acquire or shape custom eyeglass lenses, considering that
the order would most likely consist of just two different lenses, out of these materials.
Generally, Vd values above 60 are of dubious value, except in combinations of extreme
prescriptions, large lens sizes, a high wearer sensitivity to dispersion, and occupations that
involve work with high contrast elements (e.g., reading dark print on very bright white paper,
construction involving contrast of building elements against a cloudy white sky, a workplace
with recessed can or other concentrated small area lighting, etc.).
Plastic
•
•
•
•
Refractive index (nd): 1.498 (standard)
Abbe value (Vd): 59.3
Density: 1.31 g/cm³
UV cutoff: 355 nm
Plastic lenses are currently the most commonly prescribed lens, due to their relative safety,
low cost, ease of production, and outstanding optical quality. The main drawbacks are the
ease by which a lens can be scratched, and the limitations and costs of producing higher index
lenses.
Trivex™
•
•
•
•
Refractive index (nd): 1.532
Abbe value (Vd): 43 - 45 (depending on licensing manufacturer)
Density: 1.1 g/cm³ (the lightest corrective lens material in common use)
UV cutoff: 380 nm
Trivex™ is a relative newcomer that possesses the UV blocking properties and shatter
resistance of polycarbonate while at the same time offering far superior optical quality (i.e.,
higher Abbe value) and a slightly lower density. Its lower refractive index of 1.532 vs.
polycarbonate's 1.586, however, may result in slightly thicker lenses. Along with
polycarbonate and the various high-index plastics, Trivex is a lab favorite for use in rimless
frames, due to the ease with which it can be drilled as well as its resistance to cracking around
the drill holes. One other advantage that Trivex has over polycarbonate is that it can be easily
tinted.
Polycarbonate
•
•
•
•
Refractive index (nd): 1.586
Abbe value (Vd): 30
Density: 1.2 g/cm³
UV cutoff: 385 nm
Lighter weight than normal plastic. Less tendency to irritate your nose or leave red marks on
your nose where the glasses touch your nose. Polycarbonate blocks UV rays, is shatter
resistant and is used in sports glasses and glasses for children and teenagers. Because
polycarbonate is soft and will scratch easily, scratch resistant coating is typically applied after
shaping and polishing the lens. Standard polycarbonate with an Abbe value of 30 is one of the
worst materials optically, if chromatic aberration intolerance is of concern. Along with Trivex
and the high-index plastics, polycarbonate is an excellent choice for rimless eyeglasses.
High-index plastics (polyurethanes)
•
•
•
•
Refractive index (nd): 1.640 - 1.740
Abbe value (Vd): 42 - 32 (higher indexes generally result in lower Abbe values)
Density: 1.3 - 1.5 (g/cm³)
UV cutoff: 380 nm - 400 nm
High-index plastics allow for thinner lenses. The lenses may not be lighter, however, due to
the increase in density vs. mid- and normal index materials. Despite being popular with
customers, due their thinner appearance, high-index lenses also suffer from a much higher
level of chromatic aberrations due to their lower Abbe value. For people with strong
prescriptions, the significant reduction in thickness may warrant the reduction in optical
quality. Aside from thinness of the lens, another advantage of high-index plastics is their
strength and shatter resistance, although not as shatter resistant as polycarbonate. This makes
them another excellent choice for rimless eyeglasses.
Ophthalmic material property tables
Material,
Plastic
Index ABBE Specific UVB/ Reflected
(Nd) (Vd) Gravity UVA light (%)
1.50
58
1.27
100%
/
7.92
100%
CR-39® Hard
1.50
Resin
58
1.32
100%
7.97
/ 90%
Next Gen
Transitions®
Minimum
thickness
typ/min
(mm)
?/2.0
Note
1.11
100%
/
8.70
100%
?/1.0
47
1.21
100%
8.96
/ 98%
(also Vision
3456
(Kodak)?)
37
1.23
100%
/
9.52
100%
PPG Trivex™
1.53
(Average)
44
SOLA
Spectralite®
1.54
Essilor
Ormex®
1.56
Polycarbonate 1.59
30
1.20
100%
/
10.27
100%
MR-8 1.6
Plastic
1.6
41
1.30
100%
/
10.43
100%
MR-6 1.6
Plastic
1.6
36
1.34
100%
/
10.57
100%
1.22
100%
/
10.65
100%
SOLA
Finalite™
1.60
42
MR-7 1.67
Plastic
1.66
32
1.35
100%
/
12.26
100%
MR-10 1.67
Plastic
1.66
32
1.37
100%
/
12.34
100%
?/1.5
PPG,Augen,
HOYA, Thai
Optical, X-cel,
Younger
Tegra (VisionEase) Airwear
(Essilor)
FeatherWates
(LensCrafters)
Hoya EYRY
1.70
MR-174 1.74
Plastic
Material,
Glass
Crown
Glass
1.73
36
33
1.41
100%
/
13.44
100%
1.47
100%
/
14.36
100%
Index ABBE Specific UVB/ Reflected
(Nd)
(Vd) Gravity UVA light (%)
?/1.5
Hyperindex 174
(Optima)
Minimum
thickness
typ/min (mm)
Note
1.525 59
2.54
79% /
8.59
20%
PhotoGray
1.523 57
Extra®
2.41
100%
8.59
/ 97%
1.6 Glass
2.62
100%
10.68
/ 61%
Zeiss Uropal,
VisionEase, XCel
2.93
100%
13.47
/ 76%
Zeiss Tital, XCel, VisionEase,
Phillips
Zeiss Tital, XCell, Phillips,
VisionEase
Zeiss Lantal
1.7 Glass
1.604 40
1.706 30
1.8 Glass
1.800 25
3.37
100%
16.47
/ 81%
1.9 Glass
1.893 31
4.02
100%
18.85
/ 76%
Reflected light calculated using Fresnel Reflection Equation for normal waves against air on
two interfaces. This is reflection w/o AR coating.
Compilations of manufacturer material data can be found at opticampus, firstvisionmedia, and
eyecarecontacts. Additional information on branding can be found at eyetopics.
Lens coatings
Anti-reflective
The effects of an anti-reflective coating applied to an eyeglass lens
Anti-reflective coatings help to make the eye behind the lens more visible. They also help
lessen back reflections of the white of the eye as well as bright objects behind the eyeglasses
wearer (e.g., windows, lamps). Such reduction of back reflections increases the apparent
contrast of surroundings. At night, anti-reflective coatings help to reduce headlight glare from
oncoming cars, street lamps and heavily lit or neon signs.
One problem with anti-reflective coatings is that historically they have been very easy to
scratch. Newer coatings, such as Crizal® Alizé™ with its 5.0 rating and Hoya's Super
HiVision™ with its 10.9 rating on the COLTS Bayer Abrasion Test (glass averages 12-14),
try to address this problem by combining scratch resistance with the anti-reflective coating.
They also offer a measure of dirt and smudge resistance, due to their hydrophobic properties
(110° water drop contact angle for Super HiVision™ and 112° for Crizal® Alizé™).
Ultraviolet protection
A UV coating is used to reduce the transmission of light in the ultraviolet spectrum. UV-B
radiation increases the likelihood of cataracts, while long term exposure to UV-A radiation
can damage the retina. DNA damage from UV light is cumulative and irreversible. Some
materials, such as Trivex and Polycarbonate naturally block most UV light and do not benefit
from the application of a UV coating.
Scratch resistance
Highly recommended, especially for polycarbonate and softer materials, to make lenses last
longer. This is done automatically by many labs for polycarbonate and high index lenses.
Confusing corrective lens industry terminology
Spheric vs. aspheric, atoric, etc.
Lens manufacturers claim that aspheric lenses improve vision over traditional spheric lenses.
This statement could be misleading to individuals who do not know that the lenses are being
implicitly compared to "a spheric flattened away from best-form for cosmetic reasons". This
qualification is necessary since best-form spherics are always better than aspherics for an
ophthalmic lens application. Aspherics for corrective lenses are only used to attempt to
improve the degradation caused by deviating from best-form sphere resulting from making a
flatter lens for cosmetic reasons. The same applies for atoric and bi-aspheric.
It is true that aspheric lenses are used in cameras and binoculars. It would be wrong to assume
that this means aspherics/atorics are a sign of good optics in eyewear. Cameras and telescopes
use multiple lens elements and have different design criteria. Spectacles are made of only one
ophthalmic lens. The best-form spheric lens has been shown to give the best vision. In cases
where best-form is not used, such as cosmetic flattening, thinning, or wrap-around sunglasses,
an aspheric design can reduce the amount of induced optical distortions.
The problem with aspheric lenses is that they are a broad category. A lens is made of two
curved surfaces, and an aspheric lens is a lens where one or both of those surfaces is not
spherical. Further research and development is being conducted to determine if the
mathematical and theoretical benefits of aspheric lenses can actually lead to better vision
correction.
Optical aberrations of the eye lens vs. corrective lens
Optical terms are used to describe error in the eye's lens and the corrective lens. This can
cause confusion, since "astigmatism" or "ABBE" has drastically different impact on vision
depending on which lens has the error.
Astigmatism disambiguation
Astigmatism of the eye: People prescribed a sphere and a cylinder prescription have
astigmatism of the eye and can be given a toric lens to correct it.
Astigmatism of the corrective lens: This phenomenon is called lens-induced oblique
astigmatism error (OAE) or power error and is induced when the eye looks through the
ophthalmic lens at a point oblique to the optical center (OC). This may become especially
evident beyond -6D.
Example: A patient with astigmatism (or no astigmatism) of the eye and a high prescription
may notice astigmatism of the lens (OAE) when looking through the corner of their glasses.
Aspheric and atoric disambiguation
An ophthalmic "aspheric lens" specifically refers to a subclass of aspheric lens. Designs
referring to "flatter" curves are trading off optical quality for cosmetic appearance. An
aspheric lens attempts to correct the error induced by flattening the lens by using a nonspheric lens shape. Typically the design focuses on reducing the error (OAE) across the
horizontal and vertical lens axis edges. This can be primarily beneficial to farsighted
individuals, whose lenses have a thick center.
An atoric lens design refers to a lens with more complex aspheric lens design. An atoric lens
design can address error over more corners of the lens, not just the horizontal and vertical
axis.
"A toric" (two words, not "atoric") lens is a lens designed to compensate for the patients with
astigmatism in their eye. Even though this technically requires an "aspheric" lens, "aspheric"
and "atoric" are reserved for lenses which correct errors induced by cosmetic lens flattening.
http://en.wikipedia.org/wiki/Corrective_lens
INFORMATION ABOUT NEARSIGHTEDNESS
Patients with nearsightedness, or myopia, are unable to focus clearly on distant objects.
Nearsightedness occurs when light enters the eye, but focuses in front of the retina rather than
directly on it. This is typically the result of an eye that is too long or a cornea that is too steep.
Symptoms of Nearsightedness
•
•
Difficulty seeing distant objects
Squinting in order to see more clearly
Treatment Options
Nearsighted patients often wear glasses or contacts to help them see more clearly. However,
with advancements in technology, there are now alternative treatment options available
through refractive surgery. Refractive surgery options for the treatment of myopia include
LASIK (laser-assisted in situ keratomileusis) and CLEAR™ (clear lens extraction and
replacement) and more recently ICL (Implantable Collamer Lens).
LASIK
CLEAR™ - Clear Lens Extraction and Replacement
PRK
ICL
LASIK
LASIK, which stands for laser-assisted in situ keratomileusis, is a procedure that permanently
reshapes the cornea for clearer vision. LASIK is ideal for patients who want to experience
clear vision without the hassle of glasses or contacts. LASIK corrects nearsightedness,
farsightedness and astigmatism.
Before LASIK, the doctor will administer anesthetic drops to numb the eye. A lid speculum
will be used to hold the eye open and prevent the patient from blinking during the procedure.
he will create a flap in the corneal tissue using a special instrument called a microkeratome.
Patients may feel slight pressure as this flap is created.
He will then fold back the flap to reveal the underlying corneal tissue. As the patient stares
directly at a target light, the laser will gently reshape the cornea. By removing tissue from the
middle of the cornea, causing it to become flatter, nearsightedness will be corrected. This part
of the procedure typically lasts less than a minute. Once the cornea is reshaped, the flap will
be returned to its original position where it bonds securely in place without the need for
stitches.
Ideal candidates for LASIK are individuals over 21, who have had a stable prescription for at
least a year.
CLEAR™ - Clear Lens Extraction and Replacement
Offering another approach to refractive surgeryis CLEAR™ which stands for clear lens
extraction and replacement, Unlike refractive procedures that reshape the cornea, CLEAR™
replaces the eye’s natural lens with an intraocular lens.
Before surgery, the doctor will numb the eye with anesthetic drops. Once the eye is numb, he
will remove the natural lens and replace it with an intraocular lens (IOL). A tiny incision is
made in the edge of the cornea to insert the new lens. Once inserted, the intraocular lens will
be able to increase the patient’s focusing power.
Patients over the age of 40 who are not good candidates for LASIK and who are interested
invision correction may be candidates for CLEAR™.
PRK
Photo-refractive keratectomy (PRK) was the first refractive procedure performed using an
excimer laser, and is still performed today for the improvement of low to moderate
nearsightedness. This procedure is much like LASIK; however, instead of creating a corneal
flap, the doctor completely removes the outer layer of the cornea to access underlying tissue.
He then uses a laser to reshape the cornea, resulting in clearer distance vision.
PRK is associated with a longer healing time and more postoperative discomfort than LASIK
surgery. Many patients report some discomfort during the first 24 to 48 hours after surgery.
However, results are similar to those achieved by LASIK.
PRK can be an ideal procedure for patients who are not good candidates for LASIK.
Visian™ ICL
There is yet another exciting vision correction option for people with moderate to severe
nearsighted: Visian™ ICL (Implantable Collamer Lens). The Visian™ ICL is a lens that is
permanently implanted in the eye behind the iris and in front of the natural lens. It works by
bending light rays so that they focus on the retina.
Unlike contact lenses, the Visian™ ICL will never dry out, become dirty, get lost, or need to
be re-inserted in the eye each morning. It offers people who are not good candidates for laser
vision correction the freedom from having to wear glasses and contact lenses.
http://www.stlukeslasik.com/html/nearsightedness.html
INFORMATION ABOUT FARSIGHTEDNESS
Individuals with farsightedness or hyperopia are unable to see near and distant objects clearly.
This is the result of light focusing behind the retina, rather than directly on it. Individuals
suffering from farsightedness typically have an eye that is too short or a cornea that is too flat.
Symptoms of Farsightedness
•
•
•
Difficulty seeing objects up close and at a distance
Eye fatigue when reading
Eye strain resulting in headaches, burning, or a pulling sensation
Treatment Options
•
•
•
LASIK
CLEAR™
Conductive Keratoplasty
LASIK
LASIK, which stands for laser-assisted in situ keratomileusis, is a procedure that permanently
reshapes the cornea for clearer vision. LASIK is ideal for patients who want to experience
clear vision without the hassle of glasses or contacts. LASIK corrects nearsightedness,
farsightedness and astigmatism.
Before LASIK, the doctor will administer anesthetic drops to numb the eye. A lid speculum
will be used to hold the eye open and prevent the patient from blinking during the procedure.
He will create a flap in the corneal tissue using a special instrument called a microkeratome.
Patients may feel slight pressure as this flap is created.
He will then fold back the flap to reveal the underlying corneal tissue. As the patient stares
directly at a target light, the laser will gently reshape the cornea. By altering the shape of the
cornea, causing it to become steeper, farsightedness will be corrected. This part of the
procedure typically lasts less than a minute. Once the cornea is reshaped, the flap will be
returned to its original position where it bonds securely in place without the need for stitches.
Ideal candidates for LASIK are individuals over 21, who have had a stable prescription for at
least a year.
CLEAR™ - Clear Lens Extraction and Replacement
CLEAR™, which stands for clear lens extraction and replacement can correct
nearsightedness, farsightedness and astigmatism. During this procedure, the eye’s natural lens
is removed and replaced with an intraocular lens specifically chosen for each patient. This
new lens corrects the eye’s existing refractive error, for clearer vision without glasses. Most
patients notice an improvement in their vision immediately following surgery and are
typically able to return to their normal activities the very next day.
Ideal candidates for CLEAR™ are individuals over 40 who do not qualify for other refractive
procedures such as LASIK, but are ready to reduce their dependency on traditional glasses
and contacts.
Conductive Keratoplasty
Conductive keratoplasty (CK) is a procedure that can temporarily improve farsightedness. CK
involves using heat produced by radio frequency, rather than a laser, to alter the shape of the
cornea and improve vision.
When performing the procedure, the doctor uses a special pen-shaped tool that releases radio
frequency. This reduces the collagen around the edges of the cornea, causing the cornea to
steepen. The procedure only takes about 15 minutes per eye.
Most patients will notice significant improvement in their vision about a week after
undergoing CK. As the eyes continue to age, the results may fade. Therefore, patients may
require additional treatment five or ten years after their initial treatment.
Ideal candidates for CK are at least 40 years of age and have had a stable prescription for at
least a year.
http://www.stlukeslasik.com/html/farsightedness.html
INFORMATION ABOUT PRESBYOPIA
Problems with near vision often become apparent when an individual is about 40 years old.
Around this age, the eye begins to lose its focusing ability, a condition commonly referred to
as presbyopia.
Symptoms of Presbyopia
•
•
•
Difficulty reading, sewing, or seeing objects up close
Frequent headaches
Eye strain or eye fatigue after focusing on nearby objects
Presbyopia Treatment at our Florida Center
Presbyopia can be treated simply with a pair of over the counter reading glasses. However if
you are also experiencing difficulty with your distance vision, there may be a surgical
alternative for you such as CLEAR™.
CLEAR™ - Clear Lens Extraction and Replacement
Prior to surgery, the doctor will numb the eye with anesthetic drops. He will then remove the
natural lens through a tiny incision and replace it with a new intraocular lens (IOL). Once
inserted, the intraocular lens will increase the patient’s focusing power, significantly
improving vision.
If you are over the age of 40 and are interested in permanent vision correction, you may be a
candidate for clear lens extraction and replacement
http://www.stlukeslasik.com/html/presbyopia.html
Information About Astigmatism
Astigmatism occurs when the cornea, which should be shaped like a sphere, is instead shaped
like an oval. Oval-shaped corneas typically have two curves: a steeper one and a flatter one.
Therefore, when light passes through the cornea to the retina, it focuses on more than one
point in the eye, resulting in blurred vision.
Symptoms of Astigmatism
•
•
Difficulty seeing both near and distant objects without squinting
Blurred or distorted vision when reading
Treatment Options
Astigmatism commonly occurs in addition to nearsightedness or farsightedness and can be
treated with glasses, contacts, or through a refractive surgery procedure. At St. Luke’s in
Florida we offer several refractive surgery procedures to residents of Tampa, Orlando, and
surrounding cities for the treatment of astigmatism.
•
•
•
•
Astigmatic Keratotomy – AK
LASIK
Limbal Relaxing Incisions – LRI
STAAR Toric™ Intraocular Lens
Astigmatic Keratotomy - AK
Astigmatic keratotomy can be performed to reduce or eliminate astigmatism. During the
procedure, the doctor will create curved incisions along the steepest sections of the cornea.
These incisions will cause the steep areas of the cornea to flatten and take more of a spherical
shape. Therefore, light will focus more clearly on the retina, reducing blurred vision. AK may
be performed alone or in combination with other refractive procedures.
LASIK
LASIK, which stands for laser-assisted in situ keratomileusis, is a procedure that permanently
reshapes the cornea for clearer vision. LASIK is ideal for patients who want to experience
clear vision without the hassle of glasses or contacts. LASIK corrects nearsightedness,
farsightedness and astigmatism.
Before LASIK, the doctor will administer anesthetic drops to numb the eye. A lid speculum
will be used to hold the eye open and prevent the patient from blinking during the procedure.
He will will create a flap in the corneal tissue using a special instrument called a
microkeratome. Patients may feel slight pressure as this flap is created.
He will will then fold back the flap to reveal the underlying corneal tissue. As the patient
stares directly at a target light, the laser will gently reshape the cornea, correcting
astigmatism. This part of the procedure typically lasts less than a minute. Once the cornea is
reshaped, the flap will be returned to its original position where it bonds securely in place
without the need for stitches.
Ideal candidates for LASIK are individuals over 21, who have had a stable prescription for at
least a year.
Limbal Relaxing Incisions - LRI
Limbal relaxing incisions or LRIs are used to treat astigmatism. Unlike other procedures that
provide treatment directly to the cornea, limbal relaxing incisions are created in the limbus,
the thin layer that connects the cornea with the white of the eye.
After a few drops of anesthetic have been administered to the eye, the doctor places relaxing
incisions along the highest points at the edges of the cornea. These incisions allow the cornea
to relax and take a more spherical shape, thereby reducing blurred vision. Since they are made
along the edges rather than across the corneal tissue, LRIs also preserve visual quality. Few
side effects are associated with limbal relaxing incisions, and the wounds tend to heal quickly.
Individuals with astigmatism who are interested in reducing their dependency on glasses or
contacts may be ideal candidates for limbal relaxing incisions.
STAAR Toric™ Intraocular Lens
The STAAR Toric™ Intraocular Lens used both with cataract and CLEAR™ surgery is
designed to treat lenticular astigmatism, or an irregularly shaped natural lens. Some
individuals may have lenticular astigmatism in addition to or instead of corneal astigmatism.
By replacing the natural lens, the eye’s focusing power can be improved and astigmatism
corrected.
The STAAR Toric™ Intraocular Lens is foldable so it can be easily inserted into the eye. The
doctor will create a tiny incision at the edge of the cornea to insert the small, folded lens.
Once in place, the lens will gently unfold, its toric (rounded) curve counteracting irregularities
of the natural lens that cause astigmatism, allowing for clear, crisp vision.
CRYSTALENS
Crystalens is an FDA-approved intraocular lens for the visual correction of adults with or
without presbyopia, or lenses that have aged or become more rigid.
Crystalens was modeled after the human eye. Like the natural lens, it uses the eye muscle to
flex and accommodate in order to focus on objects in the environment at all
distances. Crystalens dynamically adjusts to your visual needs.
Crystalens accommodating intraocular lens is made with hinges on each side that are designed
to allow the optic, or the part of the lens that you see through, to move back and forth as you
constantly change focus on images around you. Crystalens is a small lens enabling it to flex or
bend as you focus your vision.
crystalens is:
•
The first and only FDA-approved accommodating intraocular lens
•
The only lens that uses the natural focusing ability of the eye
•
The only lens that provides a single focal point throughout a continuous range of
vision from far to near, and everything in between
Few patients with crystalens have experienced glare, halos and night vision problems.
Crystalens focuses only one image to the back of the eye, unlike a multifocal lens that projects
multiple images that are not entirely in focus, requiring your brain to "adjust" to the
differences. In addition, crystalens vision is sharp and crisp because 100% of the light rays are
available and distributed - when you need it.
http://www.stlukeslasik.com/crystalens/
EXCIMER LASER
An excimer laser (sometimes, and more correctly, called an exciplex laser) is a form of
ultraviolet laser which is commonly used in eye surgery and semiconductor manufacturing.
The term excimer is short for 'excited dimer', while exciplex is short for 'excited complex'. An
excimer laser typically uses a combination of an inert gas (Argon, krypton, or xenon) and a
reactive gas (fluorine or chlorine). Under the appropriate conditions of electrical stimulation,
a pseudo-molecule called a dimer is created, which can only exist in an energized state and
can give rise to laser light in the ultraviolet range.
The UV light from an excimer laser is well absorbed by biological matter and organic
compounds. Rather than burning or cutting material, the excimer laser adds enough energy to
disrupt the molecular bonds of the surface tissue, which effectively disintegrates into the air in
a tightly controlled manner through ablation rather than burning. Thus excimer lasers have the
useful property that they can remove exceptionally fine layers of surface material with almost
no heating or change to the remainder of the material which is left intact. These properties
make excimer lasers well suited to precision micromachining organic material (including
certain polymers and plastics), or delicate surgeries such as eye surgery (LASIK).
Excimer lasers
The first excimer laser was invented in 1970 by Nikolai Basov, V. A. Danilychev and Yu. M.
Popov, at the Lebedev Physical Institute in Moscow, using a xenon dimer (Xe2) excited by an
electron beam to give stimulated emission at 172 nm wavelength. A later improvement,
developed by many groups in 1975 was the use of noble gas halides (originally XeBr). These
groups include the United States Government's Naval Research Laboratory, the Northrop
Research and Technology Center, the Avco Everett Research Laboratory and Sandia
Laboratories
Laser action in an excimer molecule occurs because it has a bound (associative) excited state,
but a repulsive (disassociative) ground state. This is because noble gases such as xenon and
krypton are highly inert and do not usually form chemical compounds. However, when in an
excited state (induced by an electrical discharge or high-energy electron beams, which
produce high energy pulses), they can form temporarily-bound molecules with themselves
(dimers) or with halides (complexes) such as fluorine and chlorine. The excited compound
can give up its excess energy by undergoing spontaneous or stimulated emission, resulting in
a strongly-repulsive ground state molecule which very quickly (on the order of a picosecond)
disassociates back into two unbound atoms. This forms a population inversion between the
two states.
Most "excimer" lasers are of the noble gas halide type, for which the term excimer is strictly
speaking a misnomer (since a dimer refers to a molecule of two identical or similar parts):
The correct but less commonly used name for such is exciplex laser.
The wavelength of an excimer laser depends on the molecules used, and is usually in the
ultraviolet:
Excimer Wavelength Relative Power
Ar2*
126 nm
Kr2*
146 nm
F2
157 nm
Xe2*
172 & 175 nm
ArF
193 nm
10
60
KrF
248 nm
100
XeBr
282 nm
XeCl
308 nm
50
XeF
351 nm
45
CaF2
193 nm
KrCl
222 nm
Cl2
259 nm
N2
337 nm
25
5
Excimer lasers are usually operated with a pulse rate of around 100 Hz and a pulse duration of
~10 ns, although some operate as high as 8 kHz and 200 ns.
Uses
The high-power ultraviolet output of excimer lasers makes them useful for surgery
(particularly eye surgery), for lithography for semiconductor manufacturing, and for
dermatological treatment. Excimer laser light is typically absorbed within the first billionth of
a meter (nanometer) of tissue. The website howstuffworks.com states:
"The Excimer laser is incredibly precise. It has the ability to focus a beam as small as
0.25 micrometres [and] capable of removing 0.5% of a human hair's width at a time."
This quote is a bit misleading. The beam output from an excimer is in general multimode and
not of good quality when compared to other lasers. In laser drilling systems the excimer is
employed similar to a conventional light source. The accuracy comes from the imaging
system and the fact that UV light has a short wavelength.
Excimer lasers are quite large and bulky devices, which is a disadvantage in their medical
applications, although their size is rapidly decreasing with ongoing development.
http://en.wikipedia.org/wiki/Excimer_laser
PHOTOREFRACTIVE KERATECTOMY
Photorefractive keratectomy (PRK) and Laser-Assisted Sub-Epithelial Keratectomy (LASEK)
are laser eye surgery procedures intended to correct a person's vision, reducing dependency on
glasses or contact lenses. The first LASEK procedure was performed at Massachusetts Eye
and Ear Infirmary in 1996 by ophthalmologist, refractive surgeon, Dimitri Azar. The
procedure was later popularized by Camellin, who coined the term LASEK for laser epithelial
keratomileusis. LASEK and PRK permanently change the shape of the anterior central cornea
using an excimer laser to ablate (remove by vapourization) a small amount of tissue from the
corneal stroma at the front of the eye, just under the corneal epithelium. The outer layer of the
cornea is removed prior to the ablation. A computer system tracks the patient's eye position 60
to 4,000 times per second, depending on the brand of laser used, redirecting laser pulses for
precise placement. Most modern lasers will automatically center on the patient's visual axis
and will pause if the eye moves out of range and then resume ablating at that point after the
patient's eye is re-centered.
The outer layer of the cornea, or epithelium, is a soft, rapidly regrowing layer in contact with
the tear film that can completely replace itself from limbal stem cells within a few days with
no loss of clarity. The deeper layers of the cornea, as opposed to the outer epithelium, are laid
down early in life and have very limited regenerative capacity. The deeper layers, if reshaped
by a laser or cut by a microkeratome, will remain that way permanently with only limited
healing or remodelling. In LASEK, the corneal epithelium is preserved with a chemical
solution, peeled off, and replaced after the laser ablation is complete. In PRK, the epithelium
removed is discarded and allowed to regenerate. Both procedures are distinct from LASIK
(Laser-Assisted in-SItu Keratomileusis), a form of laser eye surgery where a permanent flap is
created in the deeper layers of the cornea.
PRK versus LASIK
Because PRK does not create a permanent flap in the deeper corneal layers (the LASIK
procedure involves a mechanical microkeratome using a metal blade or a femtosecond laser
microkeratome to create a 'flap' out of the outer cornea), the cornea's structural integrity is less
altered by PRK.
The LASIK process covers the laser treated area with the flap of tissue which is from 100 to
180 micrometres thick. This flap can mute the nuances of the laser ablation, whereas PRK
performs the laser ablation at the outer surface of the cornea. The use of the anti-metabolite
mitomycin can minimize the risk of post-operative haze in persons requiring larger PRK
corrections.
PRK does not involve a knife, microkeratome, or cutting laser as used in LASIK, but there
may be more pain and slower visual recovery. Unlike LASIK, PRK does not create the risk of
dislocated corneal flaps which may occur (especially with trauma), at any time after LASIK.
An evolved form of PRK is called No Touch laser vision correction. It also treats the surface
of the cornea but unlike other techniques, requires no assistance from manual surgical
instruments. It is the only technique to use exclusively an excimer laser from start to finish.
PRK eligibility
It is estimated that up to 80% of the myopic population may physically qualify as potential
PRK candidates.There are a number of basic criteria which a potential candidate should
satisfy:
•
•
•
•
•
•
•
Normal ocular health
Age 20 years or older
Stable refraction error (no noticeable change in the last year) correctable to 20/40 or
better
Between -1.50 to -7.00 diopters of Myopia
No gender restriction, with the exception of pregnancy
Realistic expectations of the final results (with a complete understanding of the
benefits, as well as the possible risks)
Pupil size 6 mm in room light
There are also some pre-existing conditions that may complicate or preclude the treatment.
•
•
•
•
Collagen vascular disease (e.g., corneal ulceration or melting)
Ocular disease (e.g., dry eye, keratoconus, glaucoma)
Systemic disorders (e.g., diabetes, rheumatoid arthritis)
History of side effects from steroids
Possible complications
Some complications of PRK include:
•
•
•
•
•
•
•
•
•
Long healing period
Pain
Glare, halos, or starburst Aberrations
Under- or over-correction
Recurrence of myopia
Corneal haze
Scarring
Reduced best corrected visual acuity
Reduced acuity in low light
PRK may be performed on one eye at a time to assess the results of the procedure and ensure
adequate vision during the healing process. Activities requiring good binocular vision may
have to be suspended between surgeries and during the sometimes extended healing periods.
A few post-PRK patients have complained of glare, halos, and starburst aberrations, which
may be the result of postoperative corneal haze that may develop during the healing process.
Using modern lasers as of the year 2005, this is quite rare after 6 months but reportedly,
symptoms have occasionally lingered longer than a year in some cases.
Predictability of the resulting refractive correction after healing is not totally exact,
particularly for those with more severe myopia. This can lead to under/over-correction of the
refractive error. In the case of the over-correction, premature presbyopia is a possibility.
In 1 to 3% of cases, loss of best corrected visual acuity (BCVA) can result, due to decentered
ablative zones or other surgical complications. PRK results in improved BCVA about twice as
often as it causes loss. Decentration is becoming less and less of a problem with more modern
lasers using sophisticated eye centering and tracking methods.
Aviator usage
Operation of an aircraft is a visually demanding activity performed in an environment that is
not always user friendly. Currently, over 50% of the civil airman population uses some form
of visual correction. Aviators considering PRK should know that clinical trials claiming
success rates of 90% or higher are based on criteria of 20/40 or better, not 20/20 or better,
uncorrected visual acuity.
Some PRK patients have reported dissatisfaction with their vision under low ambient lighting
(dusk/nighttime) conditions. Pilots who experience postoperative vision problems could be
further compromised by the variations in lighting common to the aviation environment. In
addition, exposure to intense UV radiation has been associated with late-onset corneal haze
and recurrence of myopia.
The US Federal Aviation Administration will consider applicants with PRK once they are
fully healed and stabilized, provided there are no complications and all other visual standards
are met. Pilots should be aware, however, that potential employers, such as commercial
airlines and private companies, may have policies that consider refractive surgery a
disqualifying condition. Also, civilians who wish to fly military aircraft should know that
there are restrictions on those who have had corrective surgery. The Army now permits flight
applicants who have undergone PRK or LASIK, though it still requires a standard waiver. The
Navy and Marines will routinely grant a waiver for pilots or student naval aviators to fly after
PRK, assuming no complications and acceptable vision. LASIK is currently disqualifying for
the Navy. In one study 967 of 968 naval aviators having PRK returned to duty involving
flying after the procedure. In fact, the U.S. Navy now offers free PRK surgery at the National
Naval Medical Center to Naval Academy Midshipmen who intend to pursue career paths
requiring perfect uncorrected vision, including flight school and special forces training. The
U.S. Air Force approves the use of PRK and recently approved LASIK (pilots must have
LASIK performed by Air Force Ophthalmologists at Wilford Hall Medical Center).
In the majority of patients, PRK has proven to be a safe and effective procedure for the
correction of myopia. PRK is still evolving with other countries currently using refined
techniques and alternative procedures. Many of these procedures are under investigation in
the U.S. Given that PRK is not reversible, a patient considering PRK is recommended to
contact an eye-care practitioner for assistance in making an informed decision concerning the
potential benefits and liabilities that may be specific to him or her.
http://en.wikipedia.org/wiki/Photorefractive_keratectomy
LASIK (laser-assisted in situ keratomileusis) is a type of refractive laser eye surgery
performed by ophthalmologists for correcting myopia, hyperopia, and astigmatism. The
procedure is generally preferred to photorefractive keratectomy, PRK, (also called ASA,
Advanced Surface Ablation) because it requires less time for the patient's recovery, and the
patient feels less pain, overall; however, there are instances where PRK/ASA is medically
indicated as a better alternative to LASIK.
Many patients choose LASIK as an alternative to wearing corrective eyeglasses or contact
lenses.
Technological development
The LASIK technique was made possible by the Colombian-based Spanish ophthalmologist
Jose Barraquer, who, around 1950 in his clinic in Bogotá, Colombia, developed the first
microkeratome, used to cut thin flaps in the cornea and alter its shape, in a procedure called
keratomileusis. He also provided the knowledge about how much of the cornea had to be left
unaltered to provide a stable long-term result.
Later technical and procedural developments included the RK (radial keratotomy) started in
the '70s in Russia by Svyatoslav Fyodorov and the development of PRK (photorefractive
keratectomy) in the '80s in Germany by Theo Seiler.
In 1968, at the Northrup Corporation Research and Technology Center of the University of
California, Mani Lal Bhaumik and a group of other scientists, while working on the
development of a carbon-dioxide laser, would develop the Excimer laser, where molecules
that do not normally exist come into being when xenon, argon or krypton gases are excited.
This would form the cornerstone for LASIK eye surgery. Dr. Bhaumik announced his
discovery in May of 1973 at a meeting of the Denver Optical Society of America in Denver,
Colorado. He would later patent it.
The introduction of Laser in this refractive procedure started with the developments in Laser
technology by Rangaswamy Srinivasan. In 1980, Srinivasan, working at IBM Research Lab,
discovered that an ultraviolet excimer laser could etch living tissue in a precise manner with
no thermal damage to the surrounding area. He named the phenomenon Ablative
Photodecomposition (APD). Dr. Stephen Trokel published a paper in the American Journal of
Ophthalmology in 1983, outlining the potential of using the excimer laser in refractive
surgeries.
The first patent for LASIK was granted by the US Patent Office to Gholam A. Peyman, MD
on June 20, 1989, US Patent #4,840,175, "METHOD FOR MODIFYING CORNEAL
CURVATURE", describing the surgical procedure in which a flap is cut in the cornea and
pulled back to expose the corneal bed. This exposed surface is then ablated to the desired
shape with an excimer laser, following which the flap is replaced.
Using these advances in laser technology and the technical and theoretical developments in
refractive surgery made since the 50's, LASIK surgery was developed in 1990 by Lucio
Buratto (Italy) and Ioannis Pallikaris (Greece) as a melding of two prior techniques,
keratomileusis and photorefractive keratectomy. It quickly became popular because of its
greater precision and lower frequency of complications in comparison with these former two
techniques. Today, faster lasers, larger spot areas, bladeless flap incision, and wavefrontoptimized and -guided techniques have significantly improved the reliability of the procedure
as compared to that of 1991. Nonetheless, the fundamental limitations of excimer lasers and
undesirable destruction of the eye's nerves have spawned research into many alternatives to
"plain" LASIK, including all-femtosecond correction (Femtosecond Lenticule EXtraction,
FLIVC), LASEK, Epi-LASIK, sub-Bowman’s Keratomileusis aka thin-flap LASIK,
wavefront-guided PRK, and modern intraocular lenses.
Procedure
There are several necessary preparations in the preoperative period. The operation itself is
made by creating a thin flap on the eye, folding it to enable remodeling of the tissue
underneath with laser. The flap is repositioned and the eye is left to heal in the postoperative
period.
Preoperative
Patients wearing soft contact lenses typically are instructed to stop wearing them
approximately 5 to 7 days before surgery. One industry body recommends that patients
wearing hard contact lenses should stop wearing them for a minimum of six weeks plus
another six weeks for every three years the hard contacts had been worn. Before the surgery,
the patient's corneas are examined with a pachymeter to determine their thickness, and with a
topographer to measure their surface contour. Using low-power lasers, a topographer creates a
topographic map of the cornea. This process also detects astigmatism and other irregularities
in the shape of the cornea. Using this information, the surgeon calculates the amount and
locations of corneal tissue to be removed during the operation. The patient typically is
prescribed an antibiotic to start taking beforehand, to minimize the risk of infection after the
procedure.
Operation
The operation is performed with the patient awake and mobile; however, the patient typically
is given a mild sedative (such as Valium) and anesthetic eye drops.
LASIK is performed in three steps. The first step is to create a flap of corneal tissue. The
second step is remodeling of the cornea underneath the flap with laser. Finally, the flap is
repositioned.
Flap creation
A corneal suction ring is applied to the eye, holding the eye in place. This step in the
procedure can sometimes cause small blood vessels to burst, resulting in bleeding or
subconjunctival hemorrhage into the white (sclera) of the eye, a harmless side effect that
resolves within several weeks. Increased suction typically causes a transient dimming of
vision in the treated eye. Once the eye is immobilized, the flap is created. This process is
achieved with a mechanical microkeratome using a metal blade, or a femtosecond laser
microkeratome (procedure known as IntraLASIK) that creates a series of tiny closely
arranged bubbles within the cornea. A hinge is left at one end of this flap. The flap is folded
back, revealing the stroma, the middle section of the cornea. The process of lifting and folding
back the flap can be uncomfortable.
Laser remodeling
The second step of the procedure is to use an excimer laser (193 nm) to remodel the corneal
stroma. The laser vaporizes tissue in a finely controlled manner without damaging adjacent
stroma. No burning with heat or actual cutting is required to ablate the tissue. The layers of
tissue removed are tens of micrometers thick. Performing the laser ablation in the deeper
corneal stroma typically provides for more rapid visual recovery and less pain, than the earlier
technique photorefractive keratectomy (PRK).
During the second step, the patient's vision will become very blurry once the flap is lifted.
He/she will be able to see only white light surrounding the orange light of the laser. This can
be disorienting.
Currently manufactured excimer lasers use an eye tracking system that follows the patient's
eye position up to 4,000 times per second, redirecting laser pulses for precise placement
within the treatment zone. Typical pulses are around 1 mJ of pulse energy in 10 to 20
nanoseconds.
Reposition of flap
After the laser has reshaped the stromal layer, the LASIK flap is carefully repositioned over
the treatment area by the surgeon, and checked for the presence of air bubbles, debris, and
proper fit on the eye. The flap remains in position by natural adhesion until healing is
completed.
Postoperative
Patients are usually given a course of antibiotic and anti-inflammatory eye drops. These are
continued in the weeks following surgery. Patients are usually told to sleep much more and
are also given a darkened pair of shields to protect their eyes from bright lights and protective
goggles to prevent rubbing of the eyes when asleep and to reduce dry eyes. They also have to
moisturize the eyes with preservative free tears and follow directions for prescription drops.
Patients should be adequately informed by their surgeons of the importance of proper postoperative care to minimize the risk of post-surgical complications.
Higher-order aberrations
Higher-order aberrations are visual problems not captured in a traditional eye exam which
tests only for acuteness of vision. Severe aberrations can effectively cause significant vision
impairment. These aberrations include starbursts, ghosting, halos, double vision, and a
number of other post-operative complications listed below.
Concern has long plagued the tendency of refractive surgeries to induce higher-order
aberration not correctable by traditional contacts or glasses. The advancement of LASIK
technique and technologies has helped reduce the risk of clinically significant visual
impairment after the surgery. One of the major discoveries was the correlation between pupil
size and aberrations. e result of the irregularity between the untouched part of the cornea and
the reshaped part. Daytime post-lasik vision is optimal, since the pupil is smaller than the
LASIK flap. But at night, the pupil may expand such that light passes through the edge of the
LASIK flap into the pupil which gives rise to many aberrations. There are other currently
unknown factors in addition to pupil size that also affect higher order aberrations.
In extreme cases, where ideal technique was not followed and before key advances, some
people could suffer rather debilitating symptoms including serious loss of contrast sensitivity
in poor lighting situations.
Over time, most of the attention has been focused on spherical aberration. LASIK and PRK
tend to induce spherical aberration, because of the tendency of the laser to undercorrect as it
moves outward from the center of the treatment zone. This is really a significant issue for only
large corrections. There is some thought if the lasers were simply programmed to adjust for
this tendency, no significant spherical aberration would be induced. Hence, in eyes with little
existing higher order aberrations, "wavefront optimized" LASIK rather than wavefront guided
LASIK may well be the future.
In any case, higher order aberrations are measured in µm (micrometers) on the wavescan
taken during the pre-op examination, while the smallest beam size of FDA approved lasers is
about 1000 times larger, at 0.65 mm. Thus imperfections are inherent in the procedure and a
reason why patients experience halo, glare, and starburst even with small naturally dilated
pupils in dim lighting.
Wavefront-guided LASIK
Wavefront-guided LASIK is a variation of LASIK surgery where, rather than applying a
simple correction of focusing power to the cornea (as in traditional LASIK), an
ophthalmologist applies a spatially varying correction, guiding the computer-controlled
excimer laser with measurements from a wavefront sensor. The goal is to achieve a more
optically perfect eye, though the final result still depends on the physician's success at
predicting changes which occur during healing. In older patients though, scattering from
microscopic particles plays a major role and may exceed any benefit from wavefront
correction. Hence, patients expecting so-called "super vision" from such procedures may be
disappointed. However, while unproven, surgeons claim patients are generally more satisfied
with this technique than with previous methods, particularly regarding lowered incidence of
"halos", the visual artifact caused by spherical aberration induced in the eye by earlier
methods.
Complications
A subconjunctival hemorrhage is a common and minor post-LASIK complication.
The incidence of refractive surgery patients having unresolved complications six months after
surgery has been estimated from 3% to 6%. The risk for a patient of suffering from disturbing
visual side effects like halos, double vision (ghosting), loss of contrast sensitivity (foggy
vision) and glare after LASIK depends on the degree of ametropia before the laser eye surgery
and other risk factors. For this reason, it is important to take into account the individual risk
potential of a patient and not just the average probability for all patients. The following are
some of the more frequently reported complications of LASIK
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Surgery induced dry eyes
Overcorrection or undercorrection
Visual acuity fluctuation
Halos or starbursts around light sources at night
Light sensitivity
Ghost imagesor double vision
Wrinkles in flap (striae)
Decentered ablation
Debris or growth under flap
Thin or buttonhole flap
Induced astigmatism
Corneal Ectasia
Floaters
Epithelium erosion
Posterior vitreous detachment
Macular hole
Complications due to LASIK have been classified as those that occur due to preoperative,
intraoperative, early postoperative, or late postoperative sources:
Intraoperative complications
•
•
•
The incidence of flap complications has been estimated to be 0.244%.Flap
complications (such as displaced flaps or folds in the flaps that necessitate
repositioning, diffuse lamellar keratitis, and epithelial ingrowth) are common in
lamellar corneal surgeries but rarely lead to permanent visual acuity loss; the
incidence of these microkeratome-related complications decreases with increased
physician experience. According to proponents of such techniques, this risk is further
reduced by the use of IntraLasik and other non-microkeratome related approaches,
although this is not proven and carries its own set of risks of complications from the
IntraLasik procedure.
A slipped flap (a corneal flap that detaches from the rest of the cornea) is one of the
most common complications. The chances of this are greatest immediately after
surgery, so patients typically are advised to go home and sleep to let the flap heal.
Patients are usually given sleep goggles or eye shields to wear for several nights to
prevent them from dislodging the flap in their sleep. A faster operation may decrease
the chance of this complication, as there is less time for the flap to dry.
Flap interface particles are another finding whose clinical significance is
undetermined. A Finnish study found that particles of various sizes and reflectivity
were clinically visible in 38.7% of eyes examined via slit lamp biomicroscopy, but
apparent in 100% of eyes using confocal microscopy
Early postoperative complications
•
The incidence of diffuse lamellar keratitis (DLK) also known as the Sands of Sahara
syndrome, has been estimated at 2.3%.When diagnosed and appropriately treated,
DLK resolves with no lasting vision limitation.
•
•
•
The incidence of infection responsive to treatment has been estimated at
0.4%.Infection under the corneal flap is possible. It is also possible that a patient has
the genetic condition keratoconus that causes the cornea to thin after surgery.
Although this condition is screened in the preoperative exam, it is possible in rare
cases (about 1 in 5,000) for the condition to remain dormant until later in life (the mid40s). If this occurs, the patient may need rigid gas permeable contact lenses,
Intrastromal Corneal Ring Segments (Intacs), Corneal Collagen Crosslinking with
Riboflavin or a corneal transplant.
The incidence of persistent dry eye has been estimated to be as high as 28% in Asian
eyes and 5% in Caucasian eyes. Nerve fibers in the cornea are important for
stimulating tear production. A year after LASIK, subbasal nerve fiber bundles remain
reduced by more than half. Some patients experience reactive tearing, in part to
compensate for chronic decreased basal wetting tear production.
The incidence of subconjunctival hemorrhage has been estimated at 10.5% according
to a study undertaken in China; thus results may not be generally applicable due to
racial and geographic factors).
Late postoperative complications
•
•
•
•
The incidence of epithelial ingrowth has been estimated at 0.1%.
Glare is another commonly reported complication of those who have had LASIK.
Halos or starbursts around bright lights at night are caused by the irregularity between
the lasered part and the untouched part. It is not practical to perform the surgery so
that it covers the width of the pupil at full dilation at night, and the pupil may expand
so that light passes through the edge of the flap into the pupil. In daytime, the pupil is
smaller than the edge. Modern equipment is better suited to treat those with large
pupils, and responsible physicians will check for them during examination.
Late traumatic flap dislocations have been reported 1–7 years post-LASIK.
Other
Lasik and other forms of laser refractive surgery (i.e. PRK, LASEK and Epi-LASEK) change
the dynamics of the cornea. These changes make it difficult for your optometrist and
ophthalmologist to accurately measure your intraocular pressure, essential in glaucoma
screening and treatment. The changes also affect the calculations used to select the correct
intraocular lens implant when you have cataract surgery. This is known to ophthalmologists
as "refractive surprise." The correct intraocular pressure and intraocular lens power can be
calculated if you can provide your eye care professional with your preoperative, operative and
postoperative eye measurements.
Although there have been improvements in LASIK technology, a large body of conclusive
evidence on the chances of long-term complications is not yet established. Also, there is a
small chance of complications, such as haziness, halo, or glare, some of which may be
irreversible because the LASIK eye surgery procedure is irreversible.
The incidence of macular hole has been estimated at 0.2 percent to 0.3 percent.The incidence
of retinal detachment has been estimated at 0.36 percent. The incidence of choroidal
neovascularization has been estimated at 0.33 percent. The incidence of uveitis has been
estimated at 0.18 percent
Although the cornea usually is thinner after LASIK, because of the removal of part of the
stroma, refractive surgeons strive to maintain a minimum thickness to avoid structurally
weakening the cornea. Decreased atmospheric pressure at higher altitudes has not been
demonstrated as extremely dangerous to the eyes of LASIK patients. However, some
mountain climbers have experienced a myopic shift at extreme altitudes. There are no
published reports documenting scuba diving-related complications after LASIK.
In situ keratomileusis effected at a later age increases the incidence of corneal higher-order
wavefront aberrations. Conventional eyeglasses do not correct higher order aberrations.
Microfolding has been reported as "an almost unavoidable complication of LASIK" whose
"clinical significance appears negligible."
Blepharitis, or inflammation of the eyelids with crusting of the eyelashes, may increase the
risk of infection or inflammation of the cornea after LASIK.
Myopic (nearsighted) people who are close to the age (mid- to late-forties) when they will
require either reading glasses or bifocal eyeglasses may find that they still require reading
glasses despite having undergone refractive LASIK surgery. Myopic people generally require
reading glasses or bifocal eyeglasses at a later age than people who are emmetropic (those
who see without eyeglasses), but this benefit is lost if they undergo LASIK. This is not a
complication but an expected result of the physical laws of optics. Although there is currently
no method to completely eradicate the need for reading glasses in this group, it may be
minimized by performing a variation of the LASIK procedure called "slight monovision." In
this procedure, which is performed exactly like distance-vision-correction LASIK, the
dominant eye is set for distance vision, while the non-dominant eye is set to the prescription
of the patient's reading glasses. This allows the patient to achieve a similar effect as wearing
bifocals. The majority of patients tolerate this procedure very well and do not notice any shift
between near and distance viewing, although a small portion of the population has trouble
adjusting to the monovision effect. This can be tested for several days prior to surgery by
wearing contact lenses that mimic the monovision effect.
Factors affecting surgery
Typically, the cornea is avascular because it must be transparent to function normally, and its
cells absorb oxygen from the tear film. Thus, low-oxygen-permeable contact lenses reduce the
cornea's oxygen absorption, sometimes resulting in corneal neovascularization—the growth of
blood vessels into the cornea. This causes a slight lengthening of inflammation duration and
healing time and some pain during surgery, because of greater bleeding.
Although some contact lenses (notably modern RGP and soft silicone hydrogel lenses) are
made of materials with greater oxygen permeability that help reduce the risk of corneal
neovascularization, patients considering LASIK are warned to avoid over-wearing their
contact lenses. Usually, it is recommended that they discontinue wearing contact lenses days
or weeks before the LASIK eye surgery.
Age considerations
New advances in eyesight corrective surgery are providing consumers greater choices.
Patients in their 40s or 50s who are considering LASIK surgery to improve their vision might
want to consider to be evaluated for implantable lenses as well. "Early signs of a cataract
might argue for surgery and implantation of multifocal lenses instead."
Patient satisfaction
The surveys determining patient satisfaction with LASIK have found most patients satisfied,
with satisfaction range being 92–98 percent. A meta-analysis dated March 2008 performed by
the American Society of Cataract and Refractive Surgery over 3,000 peer-reviewed articles
published over the past 10 years in clinical journals from around the world, including 19
studies comprising 2,200 patients that looked directly at satisfaction, revealed a 95.4 percent
patient satisfaction rate among LASIK patients worldwide.
Some patients with poor outcomes from LASIK surgical procedures report a significantly
reduced quality of life because of vision problems. Patients who have suffered LASIK
complications have created websites and discussion forums to educate the public about the
risks, where prospective and past patients can discuss the surgery. In 1999, Surgical Eyeswas
founded in New York City by RK patient Ron Link as a resource for patients with
complications of LASIK and other refractive surgeries. Other patient-founded websites to
assist those with complications are LaserMyEye founded in 2004 and Vision Surgery Rehab
in 2005. Most experienced and reputable clinics will do a full-dilated medical eye exam prior
to surgery and give adequate post-operative patient education care to minimize the risk of a
negative outcome.
For best results, Dr. Steven Schallhorn, an ophthalmologist who oversaw the US Navy's
refractive surgery program and whose research partly influenced the Navy's decision to allow
its aviators to get Lasik, recommends patients seek out what's called "all-laser Lasik"
combined with "wavefront-guided" software.
The FDA website on LASIK clearly states: "Before undergoing a refractive procedure, you
should carefully weigh the risks and benefits based on your own personal value system, and
try to avoid being influenced by friends that have had the procedure or doctors encouraging
you to do so."] As such, prospective patients still need to fully understand all the potential
issues and complications, as satisfaction is directly related to expectation.
http://en.wikipedia.org/wiki/LASIK
ION LASER
From left to right: 1 mW Uniphase HeNe on alignment-rig, 2 Watt Lexel 88 Argon Ion laser,
and power-supply. To the rear are hoses for water cooling.
An ion laser is a gas laser which uses an ionized gas as its lasing medium. Like other gas
lasers, ion lasers feature a sealed cavity containing the laser medium and mirrors forming a
Fabry-Perot resonator. Unlike HeNe lasers, the energy level transitions that contribute to laser
action come from ions. Because of the large amount of energy required to excite the ionic
transitions used in ion lasers, the required current is much greater, and as a result all but the
smallest ion lasers are water cooled. A small air cooled ion laser might produce, for example,
130mW of light with a tube current of 10A @ 105V. This is a total power draw over 1 kW,
which translates into a large amount of heat which must be dissipated.
Types of lasers
A mix of argon and krypton can result in a laser with output wavelength appearing as white
light.
Krypton laser
A krypton laser is an ion laser, a type of gas laser using krypton ions as a gain medium,
pumped by electric discharge. Krypton lasers are used for scientific research, or when krypton
is mixed with argon, for creation of "white-light" lasers, useful for laser light shows. Krypton
lasers are also used in medicine (eg. for coagulation of retina), for manufacture of security
holograms, and numerous other purposes.
Krypton lasers emit at several wavelengths through the visible spectrum: at 406.7 nm, 413.1
nm, 415,4 nm, 468.0 nm, 476.2 nm, 482.5 nm, 520.8 nm, 530.9 nm, 568.2 nm, 647.1 nm,
676.4 nm.
Argon laser
The Argon laser was invented in 1964 by William Bridges at Hughes Aircraft and is one of a
family of Ion lasers that use a noble gas as the active medium.
Argon lasers are used for retinal phototherapy (for diabetes), lithography, and pumping other
lasers. Argon lasers emit at several wavelengths through the visible and ultraviolet spectrum:
351 nm, 454.6 nm, 457.9 nm, 465.8 nm, 476.5 nm, 488.0 nm, 496.5 nm, 501.7 nm, 514.5 nm,
528.7 nm.
Common argon and krypton lasers are capable of emitting continual wave output of several
milliwatts to tens of watts continually. Their tubes are usually made of kovar, beryllium oxide
ceramics, or copper. In comparison with the helium-neon lasers requiring just a few
milliamps, the current used for pumping the krypton laser ranges in several amperes, as the
gas has to be ionized. The ion laser tube produces a lot of waste heat and requires active
cooling.
Gasses and gas mixtures found in ion lasers
•
•
•
Argon (argon laser)
Krypton (krypton laser)
Ar/Kr mix ("white-light" laser)
Power supplies
•
•
•
•
•
•
•
•
•
•
NPN passback like the Spectra-physics 270 supply
MOSFET switchers like the Omnichrome 150 supply
Early switchers used NPN_PNP Pairs, (i.e. American Laser or HGM Medical)
IGBT will be seen more in days to come
Switched Resistor (Spectra Physics)
Non Switched resistor (Home-made, typically a water heater element)
Water Cooled Resistor (Laser Ionics etc)
Phased SCR power supplies similar to long xenon arc lamps are used in medical lasers
to reduce expense (Coherent)
Power on Demand power supplies are used for pulsed medical ion laser systems, these
power supplies consist of a large capacitor bank charged by a switching supply to
enable multi watt lasers to run off common single phase power supplies in doctor's
offices.
A typical Air Cooled Argon Tube needs an equivalent series resistance of ~6 Ohms
when running @ 10 amps off 117V power. The plasma in an ion laser, unlike a
Helium Neon Laser, has a slightly positive resistance, but will still run away without
ballasting. This is why ion laser supplies are very difficult to design. On a large frame
laser, the plasma itself has an effective resistance of about -7 Ohms (Spectra Physics
171 Service Manual)
Models
•
•
•
•
•
Melles Griot (Omnichrome) 532 ,543,643
Lexel 75,85, 88, 95
Laser ionics 557
Spectra-Physics 161, 164 ,168, 171
Coherent Innova 90, Innova 300, Sabre, Enterprise
Applications
•
•
•
•
•
•
•
•
•
•
•
Surgical.
High speed typesetters.
Laser light shows.
DNA sequencers for DNA sequencing.
Spectroscopy experiments.
Providing the source for tunable-dye lasers.
Semiconductor mask inspection.
Semiconductor wafer inspection.
Direct write high density PCB lithography.
Fiber Bragg Grating production.
Long coherence length models can be used for holography.
Risks
•
•
BeO
electrical shock
http://en.wikipedia.org/wiki/Ion_laser
ELECTROOCULOGRAPHY
Electrooculography (EOG) is a technique for measuring the resting potential of the retina.
The resulting signal is called the electrooculogram. The main applications are in
ophthalmological diagnosis and in recording eye movements. Unlike the electroretinogram,
the EOG does not represent the response to individual visual stimuli.
Eye movement measurements: Usually, pairs of electrodes are placed either above and below
the eye or to the left and right of the eye. If the eye is moved from the center position towards
one electrode, this electrode "sees" the positive side of the retina and the opposite electrode
"sees" the negative side of the retina. Consequently, a potential difference occurs between the
electrodes. Assuming that the resting potential is constant, the recorded potential is a measure
for the eye position.
Ophthalmological diagnosis: The EOG is used to assess the function of the pigment
epithelium. During dark adaptation, the resting potential decreases slightly and reaches a
minimum ("dark trough") after several minutes. When the light is switched on, a substantial
increase of the resting potential occurs ("light peak"), which drops off after a few minutes
when the retina adapts to the light. The ratio of the voltages (i.e. light peak divided by dark
trough) is known as the Arden ratio. In practice, the measurement is similar to the eye
movement recordings (see above). The patient is asked to switch the eye position repeatedly
between two points (usually to the left and right of the center). Since these positions are
constant, a change in the recorded potential originates from a change in the resting potential.
ELECTROPHYSIOLOGY
"Current Clamp" is a common technique in electrophysiology. This is a whole-cell current
clamp recording of a neuron firing due to it being depolarized by current injection
Electrophysiology is the study of the electrical properties of biological cells and tissues. It
involves measurements of voltage change or electrical current flow on a wide variety of scales
from single ion channel proteins to whole tissues like the heart. In neuroscience, it includes
measurements of the electrical activity of neurons, and particularly action potential activity.
Definition and scope
Classical electrophysiological techniques
Classical electrophysiology techniques involve placing electrodes into various preparations of
biological tissue. The principal types of electrodes are: 1) simple solid conductors, such as
discs and needles (singles or arrays), 2) tracings on printed circuit boards, and 3) hollow tubes
filled with an electrolyte, such as glass pippettes. The principal preparations include 1) living
organisms, 2) excised tissue (acute or cultured), 3) dissociated cells from excised tissue (acute
or cultured), 4) artificially grown cells or tissues, or 5) hybrids of the above.
If an electrode is small enough (micrometres) in diameter, then the electrophysiologist may
choose to insert the tip into a single cell. Such a configuration allows direct observation and
recording of the intracellular electrical activity of a single cell. However, at the same time
such invasive setup reduces the life of the cell. Intracellular activity may also be observed
using a specially formed (hollow) glass pipette. In this technique, the microscopic pipette tip
is pressed against the cell membrane, to which it tightly adheres. The electrolyte within the
pipette may be brought into fluid continuity with the cytoplasm by delivering a pulse of
pressure to the electrolyte in order to rupture the small patch of membrane encircled by the
pipette rim (whole cell recording). Alternatively, ionic continuity may be established by
"perforating" the patch by allowing exogenous ion channels within the electrolyte to insert
themselves into the membrane patch (perforated patch recording). Finally, the patch may be
left intact (patch recording).
The electrophysiologist may choose not to insert the tip into a single cell. Instead, the
electrode tip may be left in continuity with the extracellular space. If the tip is small enough,
such a configuration may allow indirect observation and recording of the electrical activity of
a single cell, and is termed single unit recording. Depending on the preparation and precise
placement, an extracellular configuration may pick up the activity of several nearby cells
simultaneously, and this is termed multi-unit recording.
As electrode size increases, the resolving power decreases. Larger electrodes are sensitive
only to the net activity of many cells, termed local field potentials. Still larger electrodes, such
as uninsulated needles and surface electrodes used by clinical and surgical neurophysiologists,
are sensitive only to certain types of synchronous activity within populations of cells
numbering in the millions.
Other classical electrophysiological techniques include single channel recording and
amperometry.
Optical electrophysiological techniques
Optical electrophysiological techniques were created by scientists and engineers to overcome
one of the main limitations of classical techniques. Classical techniques allow observation of
electrical activity at approximately a single point within a volume of tissue. Essentially,
classical techniques singularize a distributed phenomenon. Interest in the spatial distribution
of bioelectric activity prompted development of molecules capable of emitting light in
response to their electrical or chemical environment. Examples are voltage sensitive dyes and
fluoresceing proteins.
After introducing one or more such compounds into tissue via perfusion, injection or gene
expression, the 1 or 2-dimensional distribution of electrical activity may be observed and
recorded.
Many particular electrophysiological readings have specific names:
•
•
•
•
•
•
•
Electrocardiography - for the heart
Electroencephalography - for the brain
Electrocorticography - from the cerebral cortex
Electromyography - for the muscles
Electrooculography - for the eyes
Electroretinography - for the retina
Electroantennography - for the olfactory receptors in arthropods
Intracellular recording
Intracellular recording involves measuring voltage and/or current across the membrane of a
cell. To make an intracellular recording, the tip of a fine (sharp) microelectrode must be
inserted inside the cell, so that the membrane potential can be measured. Typically, the resting
membrane potential of a healthy cell will be -60 to -80 mV, and during an action potential the
membrane potential might reach +40 mV. In 1963, Alan Lloyd Hodgkin and Andrew Fielding
Huxley won the Nobel Prize in Physiology or Medicine for their contribution to
understanding the mechanisms underlying the generation of action potentials in neurons.
Their experiments involved intracellular recordings from the giant axon of Atlantic squid
(Loligo pealei), and were among the first applications of the "voltage clamp" technique.
Today, most microelectrodes used for intracellular recording are glass micropipettes, with a
tip diameter of < 1 micrometre, and a resistance of several megaohms. The micropipettes are
filled with a solution that has a similar ionic composition to the intracellular fluid of the cell.
A chlorided silver wire inserted in to the pipet connects the electrolyte electrically to the
amplifier and signal processing circuit. The voltage measured by the electrode is compared to
the voltage of a reference electrode, usually a silver-silver chloride wire in contact with the
extracellular fluid around the cell. In general, the smaller the electrode tip, the higher its
electrical resistance, so an electrode is a compromise between size (small enough to penetrate
a single cell with minimum damage to the cell) and resistance (low enough so that small
neuronal signals can be discerned from thermal noise in the electrode tip).
Voltage clamp
The voltage clamp uses a negative feedback mechanism. The membrane potential amplifier
measures membrane voltage and sends output to the feedback amplifier. The feedback
amplifier subtracts the membrane voltage from the command voltage, which it receives from
the signal generator. This signal is amplified and returned into the cell via the recording
electrode.
The voltage clamp technique allows an experimenter to "clamp" the cell potential at a chosen
value. This makes it possible to measure how much ionic current crosses a cell's membrane at
any given voltage. This is important because many of the ion channels in the membrane of a
neuron are voltage gated ion channels, which open only when the membrane voltage is within
a certain range. Voltage clamp measurements of current are made possible by the nearsimultaneous digital subtraction of transient capacitive currents that pass as the recording
electrode and cell membrane are charged to alter the cell's potential. (See main article on
voltage clamp.)
Current clamp
The current clamp technique records the membrane potential by injecting current into a cell
through the recording electrode. Unlike in the voltage clamp mode, where the membrane
potential is held at a level determined by the experimenter, in "current clamp" mode the
membrane potential is free to vary, and the amplifier records whatever voltage the cell
generates on its own or as a result of stimulation. This technique is used to study how a cell
responds when electrical current enters a cell; this is important for instance for understanding
how neurons respond to neurotransmitters that act by opening membrane ion channels.
Most current-clamp amplifiers provide little or no amplification of the voltage changes
recorded from the cell. The "amplifier" is actually an electrometer, sometimes referred to as a
"unity gain amplifier"; its main job is to change the nature of small signals (in the mV range)
produced by cells so that they can be accurately recorded by low-impedance electronics. The
amplifier increases the current behind the signal while decreasing the resistance over which
that current passes. Consider this example based on Ohm's law: a voltage of 10 mV is
generated by passing 10 nanoamperes of current across 1 MΩ of resistance. The electrometer
changes this "high impedance signal" to a "low impedance signal" by using a voltage follower
circuit. A voltage follower reads the voltage on the input (caused by a small current through a
big resistor). It then instructs a parallel circuit that has a large current source behind it (the
electrical mains) and adjusts the resistance of that parallel circuit to give the same output
voltage, but across a lower resistance.
The patch-clamp technique
The cell-attached patch clamp uses a micropipette attached to the cell membrane to allow
recording from a single ion channel.
This technique was developed by Erwin Neher and Bert Sakmann who received the Nobel
Prize in 1991. Conventional intracellular recording involves impaling a cell with a fine
electrode; patch-clamp recording takes a different approach. A patch-clamp microelectrode is
a micropipette with a relatively large tip diameter. The microelectrode is placed next to a cell,
and gentle suction is applied through the microelectrode to draw a piece of the cell membrane
(the 'patch') into the microelectrode tip; the glass tip forms a high resistance 'seal' with the cell
membrane. This configuration is the "cell-attached" mode, and it can be used for studying the
activity of the ion channels that are present in the patch of membrane. If more suction is now
applied, the small patch of membrane in the electrode tip can be displaced, leaving the
electrode sealed to the rest of the cell. This "whole-cell" mode allows very stable intracellular
recording. A disadvantage (compared to conventional intracellular recording with sharp
electrodes) is that the intracellular fluid of the cell mixes with the solution inside the recording
electrode, and so some important components of the intracellular fluid can be diluted. A
variant of this technique, the "perforated patch" technique, tries to minimise these problems.
Instead of applying suction to displace the membrane patch from the electrode tip, it is also
possible to withdraw the electrode from the cell, pulling the patch of membrane away from
the rest of the cell. This approach enables the membrane properties of the patch to be analysed
pharmacologically.
Sharp electrode technique
In situations where one wants to record the potential inside the cell membrane with minimal
effect on the ionic constitution of the intracellular fluid a sharp electrode can be used. These
micropipets (electrodes) are again like those for patch clamp pulled from glass capillaries, but
the pore is much smaller so that there is very little ion exchange between the intracellular
fluid and the electrlolyte in the pipete. The resistance of the electrode in 10s or 100s of MΩ in
this case. Often the tip of the electrode is filled with various kinds of dyes like Lucifer yellow
to fill the cells recorded from, for later confirmation of their morphology under a microscope.
The dyes are injected by applying a positive or negative, DC or pulsed voltage to the
electrodes depending on the polarity of the dye.
Extracellular recording
Single Unit recording
An electrode introduced into the brain of a living animal will detect electrical activity that is
generated by the neurons adjacent to the electrode tip. If the electrode is a microelectrode,
with a tip size of about 1 micrometre, the electrode will usually detect the activity of at most
one neuron. Recording in this way is generally called "single unit" recording. The action
potentials recorded are very like the action potentials that are recorded intracellularly, but the
signals are very much smaller (typically about 1 mV). Most recordings of the activity of
single neurons in anesthetized animals are made in this way, and all recordings of single
neurons in conscious animals. Recordings of single neurons in living animals have provided
important insights into how the brain processes information. For example, David Hubel and
Torsten Wiesel recorded the activity of single neurons in the primary visual cortex of the
anesthetized cat, and showed how single neurons in this area respond to very specific features
of a visual stimulus. Hubel and Wiesel were awarded the Nobel Prize in Physiology or
Medicine in 1981. If the electrode tip is slightly larger, then the electrode might record the
activity generated by several neurons. This type of recording is often called "multi-unit
recording", and is often used in conscious animals to record changes in the activity in a
discrete brain area during normal activity. Recordings from one or more such electrodes
which are closely spaced can be used to identify the number of cells around it as well as
which of the spikes come from which cell. This process is called spike sorting and is suitable
in areas where there are identified types of cells with well defined spike characteristics. If the
electrode tip is bigger still, generally the activity of individual neurons cannot be
distinguished but the electrode will still be able to record a field potential generated by the
activity of many cells.
Field potentials
A schematic diagram showing a field potential recording from rat hippocampus. At the left is
a schematic diagram of a presynaptic terminal and postsynaptic neuron. This is meant to
represent a large population of synapses and neurons. When the synapse releases glutamate
onto the postsynaptic cell, it opens ionotropic glutamate receptor channels. The net flow of
current is inward, so a current sink is generated. A nearby electrode (#2) detects this as a
negativity. An intracellular electrode placed inside the cell body (#1) records the change in
membrane potential that the incoming current causes.
Extracellular field potentials are local current sinks or sources that are generated by the
collective activity of many cells. Usually a field potential is generated by the simultaneous
activation of many neurons by synaptic transmission. The diagram to the right shows
hippocampal synaptic field potentials. At the right, the lower trace shows a negative wave that
corresponds to a current sink caused by positive charges entering cells through postsynaptic
glutamate receptors, while the upper trace shows a positive wave that is generated by the
current that leaves the cell (at the cell body) to complete the circuit. For more information, see
local field potential.
Amperometry
Amperometry uses a carbon electrode to record changes in the chemical composition of the
oxidized components of a biological solution. Oxidation and reduction is accomplished by
changing the voltage at the active surface of the recording electrode in a process known as
"scanning". Because certain brain chemicals lose or gain electrons at characteristic voltages,
individual species can be identified. Amperometry has been used for studying exocytosis in
the neural and endocrine systems. Many monoamine neurotransmitters, e.g., norepinephrine
(noradrenalin), dopamine, serotonin (5-HT), are oxidizable. The method can also be used with
cells that do not secrete oxidizable neurotransmitters by "loading" them with 5-HT or
dopamine.
Planar patch clamp
Planar patch clamp is a novel method developed for high throughput electrophysiology.
Instead of positioning a pipette on an adherent cell, cell suspension is pipetted on a chip
containing a microstructured aperture.
Schematic drawing of the classical patch clamp configuration. The patch pipette is moved to
the cell using a micromanipulator under optical control. Relative movements between the
pipette and the cell have to be avoided in order to keep the cell-pipette connection intact.
In planar patch configuration the cell is positioned by suction - relative movements between
cell and aperture can then be excluded after sealing. An Antivibration table is not necessary.
A single cell is then positioned on the hole by suction and a tight connection (Gigaseal) is
formed. The planar geometry offers a variety of advantages compared to the classical
experiment: - it allows for integration of microfluidics, which enables automatic compound
application for ion channel screening. - the system is accessible for optical or scanning probe
techniques - perfusion of the intracellular side can be performed.
The Bioelectric Recognition Assay (BERA)
The Bioelectric Recognition Assay (BERA) is a novel method for measuring changes in the
membrane potential of cells immobilized in a gel matrix. Apart from the increased stability of
the electrode-cell interface, immobilization preserves the viability and physiological functions
of the cells. BERA is primary used in biosensor applications in order to assay analytes which
can interact with the immobilized cells by changing the cell membrane potential. In this way,
when a positive sample is added to the sensor, a characteristic, ‘signature-like’ change in
electrical potential occurs. BERA has been used for the detection for human viruses (Hepatitis
B and C viruses, herpes viruses) and veterinary disease agents (foot and mouth disease virus,
prions, blue tongue virus) and plants (tobacco and cucumber viruses) in a highly specific,
rapid (1-2 minutes), reproducible and cost-efficient fashion. The method has also been used
for the detection of environmental toxins, such as herbicides and the determination of very
low concentrations of superoxide anion in clinical samples. A recent advance in the evolution
of the BERA technology was the development of a technique called Molecular
Identification through Membrane Engineering (MIME). This technique allows for
building cells with absolutely defined specificity against virtually any molecule of interest, by
embedding thousand of artificial receptors into the cell membrane.
Electroretinography is used to measure the electrical responses of various cell types in
the retina, including the light-sensitive cells (rods and cones) and the ganglion cells.
Electrodes are placed on the cornea and the skin near the eye. During a recording, the
patient is watching a standardized stimulus and the resulting signal is interpreted in
terms of its amplitude (voltage) and time course. Stimuli include flashes (flash ERG) and
reversing checkerboard patterns (pattern ERG). Applications are predominantly in
Optometry and ophthalmology, where the electroretinogram (ERG) is used for the
diagnosis of various retinal diseases:
•
•
•
•
Retinitis pigmentosa and related hereditary degenerations
Retinitis pigmentosa sine pigmento
Retinitis punctata albescens
Leber's congenital amaurosis
•
•
•
•
•
•
•
•
•
Choroideremia
Gyrate atrophy of the retina and choroid
Goldman-Favre syndrome
Congenital stationary night blindness - normal a-wave indicates normal
photoreceptors; absent b-wave indicates abnormality in the bipolar cell region.
X-linked juvenile retinoschisis
Achromatopsia
Cone dystrophies
Disorders mimicking retinitis pigmentosa
Usher Syndrome
The multifocal ERG is used to record separate responses for different retinal locations.
Electroretinograms can be broken down into three components: an initial a-wave, caused by
extracellular ionic currents generated by photoreceptors during phototransduction, the b-wave,
which corresponds to bipolar cell activity, and the later c-wave, which is generated by the
retinal pigment epithelium and Müller cells. Depending on the species the ERG is taken from,
the c-wave may be positive, negative, or absent in part or in whole.
http://en.wikipedia.org/wiki/Electroretinography
VISUAL EVOKED POTENTIAL
A visual evoked potential (VEP) is an evoked potential caused by sensory stimulation of a
subject's visual field and is observed using an electroencephalography. Commonly used visual
stimuli are flashing lights, or checkerboards on a video screen that flicker between black on
white to white on black (invert contrast).
Visual evoked potentials are very useful in detecting blindness in patients that cannot
communicate, such as babies or non-human animals. If repeated stimulation of the visual field
causes no changes in EEG potentials, then the subject's brain is probably not receiving any
signals from his/her eyes. Other applications include the diagnosis of optic neuritis, which
causes the signal to be delayed. Such a delay is also a classic finding in Multiple Sclerosis.
Visual evoked potentials are furthermore used in the investigation of basic functions of visual
perception.
The term "visual evoked potential" is used interchangeably with "visually evoked potential".
It usually refers to responses recorded from the occipital cortex. Sometimes, the term "visual
evoked cortical potential" (VECP) is used to distinguish the VEP from retinal or subcortical
potentials.
The multifocal VEP is used to record separate responses for visual field locations.
Some specific VEPs are:
•
•
•
•
•
Sweep visual evoked potential
Binocular visual evoked potential
Chromatic visual evoked potential
Hemi-field visual evoked potential
Flash visual evoked potential
•
•
•
•
•
•
LED Goggle visual evoked potential
Motion visual evoked potential
Multifocal visual evoked potential
Multi-channel visual evoked potential
Multi-frequency visual evoked potential
Stereo-elicited visual evoked potential
EYE EXAM
Your eye exam begins with a thorough investigation of the lids, lashes,
conjunctiva, sclera and cornea – the external surfaces. Using a microscope and a
bright light, the doctor will move in for a closer look at the anterior chamber, iris
and crystalline lens. The iris is very similar to the shutter of a camera. When you take a
picture on a bright sunny day, the shutter becomes smaller, allowing less light to enter.
Likewise, your pupil becomes smaller when we shine a bright light at your eye, making it
very difficult to peer inside. That’s where the dilating drops come in.
Dilating drops work on one of two principles: they either stimulate the iris muscle that opens
the pupil (the dilator), or prevent action of the iris muscle that closes the pupil (the sphincter).
After the drops take effect, your doctor can get a much better view of your retina, optic nerve
and vessels in the back of the eye. This is a very important part of your preventative eye care
as well as some eye surgeries. From this simple step, we are able to gather a lot of important
information about your eyes. In fact, some systemic diseases such as hypertension and
diabetes are first discovered during the dilated eye exam.
Some dilating drops also prevent accommodation. The natural lens is able to accommodate or
adjust the eye’s focus until about the age of 40. Children and young adults are especially
good at this, and their ability to accommodate sometimes prevents the doctor from getting an
accurate refraction for glasses. That’s why young eyes are often dilated for a “wet” refraction
so the doctor can get a true picture of what the child’s prescription really is.
There are a few things you can do to make your visit a bit more comfortable:
1. Don’t plan any activities after your appointment that require crisp vision. (Plan to
read the stock market page another time.)
2. Bring along a pair of dark sunglasses for the ride home. Don’t worry if you forget
yours, just ask for a disposable pair as you check out.
3. If you know you’ll have trouble seeing to drive home (even with the sunglasses),
please bring a friend.
4. Bring a newspaper, book or magazine with larger print to read after the drops begin to
work.
http://www.stlukeseye.com/eyeq/Dilation.asp
Intraocular Pressure (IOP)
The intraocular pressure, an important part of any eye exam, is measured with a special
instrument called a tonometer. The IOP is determined by a balance of the eye’s production
and drainage of aqueous (the clear fluid inside the eye) from the anterior
chamber into the trabecular meshwork. If the IOP is elevated, it can cause
pressure within the eye to increase and damage the optic nerve. Since abnormal
pressures usually don’t cause symptoms, it’s very important to have the
pressure checked regularly.
http://www.stlukeseye.com/eyeq/IOP.asp
Ophthalmoscopy
An ophthalmoscope is an instrument used to examine the retina and vitreous.
Ophthalmoscopy requires dilating the pupils with drops to give the doctor the best view inside
the eye.
There are two types of ophthalmoscopes: direct and indirect. The direct is a hand-held
instrument with a battery powered light source. It also has a series of lenses that can be dialed
in to focus the doctor’s view of the retina. The direct ophthalmoscope is useful for examining
the central retina.
The indirect ophthalmoscope can be used to examine the entire retina. This instrument is
worn on the doctor’s head. While looking through the instrument’s magnifying glasses, a
special lens is placed in front of the patient’s eye, allowing the doctor to see the retina clearly.
http://www.stlukeseye.com/eyeq/Ophthalmoscopy.asp
Prism testing
Prisms bend light, changing the object’s position. Because of
this property, they are commonly used to detect and measure
strabismus (turned or crossed eye). While the patient is
staring at an object, prisms of increasing strength are placed
over the turned eye until it is aligned with the fellow eye.
The stronger the prism that is required to align the eyes, the
greater the eye turn.
http://www.stlukeseye.com/eyeq/PrismTesting.asp
Pupil light reflexes (Hirschberg and Krimsky Tests)
Both of these tests are performed by simply shining a bright light into the patient’s eyes and
looking at the light in the pupils. When there are no alignment problems, the light reflection
will be in approximately the same position in both pupils. However, if the patient has
strabismus, the light will appear off-center in the crossed eye.
This test is especially useful when
examining young children.
Parents can quickly screen their
children at home with this test.
Shine a bright light at the child’s
eyes while standing about 6 feet
away. Is the light reflex
positioned equally in the child’s
pupils? If the light falls in a different spot in one pupil compared to the other, consult with an
ophthalmologist. Beware however, that some eye turns are very subtle and may only be
detected by an eye care professional.
http://www.stlukeseye.com/eyeq/PupilReflexes.asp
Refraction
The refraction is a vision test that determines your best visual acuity with corrective lenses. It
can be done with computerized equipment, but typically an instrument called a phoropter is
used. The phoropter holds corrective lenses that are positioned in front of your eye. While
looking at the eye chart through the phoropter, the technician or doctor will adjust the lenses
until the chart appears the clearest possible.
http://www.stlukeseye.com/eyeq/Refraction.asp
Slit Lamp Examination
The slit lamp is a microscope with a light attached that allows the doctor to examine your eye
under high magnification. This instrument is primarily used to view the anterior structures of
the eye such as the cornea, iris, and lens. However, with special lenses, it is possible to
examine the vitreous and the back of the eye as well.
The instrument’s name is derived from its adjustable light beam. By changing the width of
the beam, the doctor can gather important detail about each eye structure. The next time you
accompany a family member to an eye exam, ask to look in the slit lamp. You’ll be amazed
at what you see!
http://www.stlukeseye.com/eyeq/SlitLamp.asp
Visual Field
The visual field is used to test and monitor peripheral vision. It gives the doctor very
important information about the neurological function of the retina, optic nerve, and brain.
This test is usually ordered to monitor certain eye diseases such as glaucoma, and also as a
screening test prior to surgery.
Visual field tests come in different forms, but most have a
white bowl with a small fixation light in the center. The most
sophisticated ones are computerized. After your pupils have
been dilated, you will be comfortably seated in front of the
instrument. The trick to the test is to stare straight ahead as
lights flash in the periphery. A button is available to press
each time a light appears. A technician is available
throughout the test should you have a question or need to
pause for a break.
Afterward, the computer analyzes the data and prints a chart of the results. Your visual field
results are organized in your record so that your doctor can monitor your progress.
http://www.stlukeseye.com/eyeq/VisualField.asp
Have you ever wondered what 20/20 means?
The vision test is one of the simplest yet most important components of the eye exam. In
order for eye doctors to compare results, it’s always done at a standardized distance of
twenty feet. Old-fashioned offices had rooms that were twenty feet long. Today, mirrors
are used to reflect the image so the room can be shorter; but the image still looks like it is
twenty feet away. The charts are standardized too and doctors around the world use the
same basic format. But what do those numbers mean?
Each line of the eye chart is assigned a notation in the form of a fraction that represents your
visual acuity. The numerator is the distance in feet the patient is from the eye chart. The
denominator represents the distance an eye with “normal” vision can read the same line.
Interpreting the numbers is simple. If you can read the 20/40 line, you’re able to see at 20
feet what a normal eye could see at 40. And if your vision is 20/16? You’re above average
because you can see an object from 20 feet that a normal eye sees at 16!
http://www.stlukeseye.com/eyeq/Vision.asp
Contrast Sensitivity
Contrast sensitivity testing is method used to assess the quality of
vision. It differs from typical visual acuity testing in that it
simulates "real-world" circumstances. Routine visual acuity testing
measures eyesight under best possible conditions. It does not reflect
the difficulties one might experience when driving at night, or
trying to read a sign on a cloudy, overcast afternoon.
The test is performed by showing the patient a series of stripes or bars that slant in different
directions. The patient must identify which way each series of stripes is tilted. As the test
progresses, the bars become thinner and lighter. Patients with excellent contrast sensitivity
can determine the direction very light, thin bars are slanted.
This is particularly useful for measuring visual acuity in patients who report difficulty with
their vision, yet see well on the conventional eye chart.
http://www.stlukeseye.com/eyeq/ContrastSensitivity.asp
Ultrasound
Ultrasound utilizes sound waves to form an image of the eye. It works in a very similar
manner that sonar is used to "view" the ocean floor. High frequency sound waves (out of the
range of the human ear) are emitted from a probe. The sound waves travel through eye,
reflect from ocular structures back to the transducer inside the probe. The transducer receives
the sound waves and converts them into the image that appears on the examiner's screen.
Ophthalmic ultrasound is used to measure the parts of the eye, document pathology such as
tumors, and examine inside the eye. The sound frequency emitted from the probe determines
the type of image formed on the screen.
The two most common types of ultrasound used in ophthalmology are A-Scans and B-Scans.
A-Scan
A-Scan is a one-dimensional display of sound waves. Each time a sound wave hits a structure
in the eye, a spike is formed on the examiner's screen. The height and spacing between each
of the echoes provides the examiner with valuable information. A-Scans are most commonly
used to measure the eye length to determine the appropriate intraocular lens for cataract
surgery.
B-Scan
B-Scan is used to create a two-dimensional, cross-section view of the eye. Multiple sound
waves are emitted from the probe allowing the examiner to visualize structures within the
eye.
This instrument is extremely valuable when the doctor's view inside the eye is obstructed by
blood, an extremely dense cataract, or other cloudy media.
http://www.stlukeseye.com/eyeq/ultrasound.asp
Amsler Grid
This simple screening test is used to assess the macula (the center of
the retina). The Amsler Grid consists of evenly spaced horizontal
and vertical lines printed on black or white paper. A small dot is
located in the center of the grid for fixation. While staring at the dot, the patient looks for
wavy lines and missing areas of the grid. This test is especially helpful for monitoring vision
at home.
The doctor is especially interested in the following when testing vision with the Amsler Grid:
•
•
•
Are you able to see the corners and sides of the square?
Do you see any wavy lines?
Are there any holes or missing areas?
If the lines of grid do not appear straight and parallel or there are missing areas, the doctor
will examine the back of the eye (macula) very closely. This test is frequently given to
patients for home use to monitor macular degeneration. When using the test at home, notify
the doctor if any changes in the appearance of the Amsler Grid are detected.
http://www.stlukeseye.com/eyeq/amsler.asp
Fluorescein angiogram (FA)
Fluorescein angiography (fluorescein - the type of dye that is used;
angiogram - a study of the blood vessels) is an extremely valuable test
that provides information about the circulatory system and the condition
of the back of the eye. FAs are useful for evaluating many eye diseases
that affect the retina.
Retinal photograph of a
patient complaining of
decreased vision.
Fluorescein angiogram
indicating fluid leakage
within the retina
The test is performed by injecting a special dye, called fluorescein, into a vein in the arm. In
just seconds, the dye travels to the blood vessels inside the eye. A camera equipped with
special filters that highlight the dye is used to photograph the fluorescein as it circulates
though the blood vessels in the back of the eye. If there are any circulation problems,
swelling, leaking or abnormal blood vessels, the dye and its patterns will reveal these in the
photographs. The doctor can then make a determination as to the diagnosis, and possible
treatment options for the patient.
In many cases, these photos are taken with a digital camera system, allowing the physician to
interpret the results immediately.
http://www.stlukeseye.com/eyeq/FluoresceinAngiogram.asp
Indocyanine Green Dye
Study
The "hot spot" is indicated
by the white
(hyperfluorescent) area.
(arrow)
An Indocyanine Green study
(ICG) is a special dye test used
to evaluate the circulatory
system of the choroid, the layer
just behind the retina. ICG
reacts to light with a longer
wavelength than fluorescein
dye, allowing the doctor to
pinpoint the location of leaking
vessels deeper within the eye
that may not be apparent with
fluorescein angiography.
After the ICG is injected into
the patient’s arm, it travels through the bloodstream to the eye in about 15 to 20 seconds.
Once it reaches the eye, it illuminates the leaking vessels or “hot spots” in the choroid.
Using a digital camera equipped with a special filter, the retinal photographer takes photos
as the dye travels through the vessels in the eye.
The doctor interprets the digital photos and determines whether treatment is needed. If there
is an active leak, the photos serve as a guide to seal the vessel with laser. The ICG study
helps the doctor target the leak with greater accuracy, reducing the risk of damage to
surrounding retinal tissue.
The risk of allergic reaction to the dye is very minimal; however, it cannot be performed if
the patient is allergic to shellfish or iodine.
http://www.stlukeseye.com/eyeq/icg.asp
Glaucoma test
The Arden Screening Test
The Arden Screening Test is a simplified version of a more complex test developed by Dr.
Arden at Moorfield’s Eye Hospital in England.
Glaucoma research has shown that glaucoma
affects the color vision (especially blues and
yellows) before it can be identified with an eye
exam or visual field test. Dr. Arden developed a
special color vision test to help identify and treat
glaucoma patients as early as possible.
Scott Brodie, MD, an eye surgeon from New York,
designed a simplified version of this color test that
can be completed in just minutes. For the Arden Screening Test, the patient is seated in
front of a computer screen and a series of colored circles are displayed that have a break, or
missing area on contrasting backgrounds. While looking straight ahead, the patient
identifies the location of the break with her peripheral vision. As the test progresses, the
contrast between the circle and the background slowly decreases until patient can no longer
identify the break.
This test is very important when determining whether glaucoma treatment is appropriate. St.
Luke’s Cataract & Laser Institute is one of the few facilities in the country to offer the
Arden Screening Test. This test enables us to separate patients who have elevated pressures
or other suspect glaucoma signs that are normal from those who truly have the disease and
require treatment.
http://www.stlukeseye.com/eyeq/arden.asp
Fundus Photography
Don’t be surprised if someday, your eye doctor orders photographs of the
back of your eye. These pictures are necessary to document the health of the
optic nerve, vitreous, macula, retina and its blood vessels. The photographs
are used for comparison, documentation, and sometimes to diagnose certain
eye conditions.
Because fundus photography is a highly specialized form of medical imaging,
it can’t be done with an ordinary camera. It requires a customized camera
that is mounted to a microscope with intricate lenses and mirrors. These high-powered lenses
are designed so the photographer can visualize the back of the eye by focusing light through
the cornea, pupil and lens.
Before beginning, the pupil is dilated with drops. Otherwise, it would automatically constrict
from the bright light of the camera flash. The patient is asked to stare at a fixation device so
the eyes are still. While the photographer is taking the pictures, the patient will see a series of
bright flashes. The entire process usually takes approximately five to ten minutes.
http://www.stlukeseye.com/eyeq/FundusPhotography.asp
Gonioscopy
This test is performed on patients who
have glaucoma or when the disease is
suspected. A special mirrored contact
lens is used to allow the doctor to
examine the structures in the front of the
eye. With this lens, the doctor can assess
the eye’s drainage system.
http://www.stlukeseye.com/eyeq/gonioscopy.asp
Visual Field
The visual field is used to test and monitor peripheral vision.
It gives the doctor very important information about the
neurological function of the retina, optic nerve, and brain.
This test is usually ordered to monitor certain eye diseases
such as glaucoma, and also as a screening test prior to
surgery.
Visual field tests come in different forms, but most have a
white bowl with a small fixation light in the center. The most
sophisticated ones are computerized. After your pupils have
been dilated, you will be comfortably seated in front of the
instrument. The trick to the test is to stare straight ahead as
lights flash in the periphery. A button is available to press each time a light appears. A
technician is available throughout the test should you have a question or need to pause for a
break.
Afterward, the computer analyzes the data and prints a chart of the
results. Your visual field results are organized in your record so
that your doctor can monitor your progress.
http://www.stlukeseye.com/eyeq/VisualField.asp
Corneal topography
Of all the technology currently available, corneal topography provides the most detailed
information about the curvature of the cornea. Using a very sophisticated computer and
software, thousands of measurements are taken and analyzed in just seconds. The computer
generates a color map from the data. This information is useful to evaluate and correct
astigmatism, monitor corneal disease, and detect irregularities in the corneal shape.
The map is interpreted much like other topography maps. The cool shades of blue and green
represent flatter areas of the cornea, while the warmer shades of orange and red and represent
steeper areas. This corneal map allows the physician to formulate a “3-D” perspective of the
cornea’s shape. Measuring astigmatism is important for planning refractive surgery, fitting
contact lenses, and calculating intraocular lens power.
http://www.stlukeseye.com/eyeq/topography.asp
Keratometry
Keratometry measures the corneal curvature. It is performed for similar reasons as
topography, but rather than mapping the entire corneal surface, two curves are measured – the
steepest and the flattest. These measurements give the doctor information about the cornea’s
curvature, focusing power, and whether astigmatism is present.
Some of the uses of keratometry include calculating the intraocular lens power for cataract
surgery, fitting contact lenses and monitoring the corneal curvature after surgery.
http://www.stlukeseye.com/eyeq/keratometry.asp
Pachymetry
The pachymeter is an instrument that measures the thickness of the cornea. It is useful in
monitoring the progression of certain disorders that cause the cornea to become thickened (or
filled with water), resulting in a loss of vision. Pachymetry is also performed to determine
whether the cornea is strong enough for procedures such as LASIK.
http://www.stlukeseye.com/eyeq/pachymetry.asp
Schirmer Test
This test is used to assess tear production and is helpful in treating dry eye syndrome. Tiny
paper tabs are inserted in the lower lids and removed after a few minutes. When the tab is
removed, the dampened area is measured in millimeters. This helps the doctor determine the
presence or extent of a dry eye condition.
http://www.stlukeseye.com/eyeq/schirmer.asp
Specular Microscopy/Photography
This test is used to monitor the number, density, and quality of endothelial cells that line the
back of the cornea. A microscope magnifies the cells thousands of times and the image is
captured with a camera or video camera. The number of cells within one square millimeter
are counted and recorded. The endothelium of a young, ten-year-old, healthy cornea has
approximately 3,500 cells in each square millimeter. Normal aging causes the cells to
gradually decrease over time. By age 60, most people have approximately 2,500 cells per
square millimeter.
http://www.stlukeseye.com/eyeq/SpecularMicroscopy.asp
Stereopsis Test
This test helps the examiner evaluate the quality of the patient’s depth perception. While
wearing special polarized glasses, the patient looks at a series of 3-D objects that range from
being very raised to nearly flat. In each series, the patient is asked to select the object with the
greatest 3-D effect.
http://www.stlukeseye.com/eyeq/Stereopsis.asp
Worth-4 Dot Test (depth perception test)
For this test, the patient wears glasses with one green lens and one red. The patient looks
at a target with 2 green dots, 1 red dot, and 1 white dot. Depending on the number and
color of lights the patient sees, the examiner can determine whether there is normal fusion
or if one eye is being suppressed (ignored).
http://www.stlukeseye.com/eyeq/Worth4Dot.asp
Glare Testing
One of the earliest symptoms of cataracts is glare at night. Glare occurs when light enters the
eye and bounces off an opacity such as a cataract. Most cataract patients first notice glare
when looking at headlights.
The glare test is used to assess visual function when looking at bright lights. While looking
at an eye chart, lights are introduced that simulate the effect of bright sunlight or nighttime
glare. The results of the test are important when considering cataract surgery. This is
because some patients without a significant decrease in vision under normal lighting
conditions notice that their vision drops considerably in sunlight or when looking at lights.
http://www.stlukeseye.com/eyeq/glare.asp
Potential Acuity Tests
PAM (Potential Acuity Meter) also known as the Guyton
Super Pinhole
These work on different principles, but both are helpful in assessing retinal function. The
PAM projects an eye chart directly on the retina and bypasses the cataract. This allows the
examiner to test the visual acuity without interference from the cloudy lens. For the Super
Pinhole, the patient looks at a special chart through a disc with tiny holes. This allows the
patient to isolate tiny clear spots in the cataract.
When considering cataract surgery, these tests help the doctor determine the potential visual
acuity if the cataract was removed. Testing potential acuity is especially helpful when
considering cataract surgery for patients with retinal disease such as macular degeneration.
By performing this simple test, the amount of vision loss that can be attributed to the
degeneration vs. the cataract can be determined.
http://www.stlukeseye.com/eyeq/pam.asp