* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Novel, Secondary Sensory Cell Organ in Ascidians: In Search of the
Survey
Document related concepts
Transcript
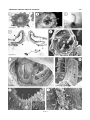
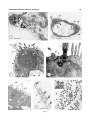
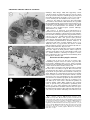
THE JOURNAL OF COMPARATIVE NEUROLOGY 461:236 –249 (2003) Novel, Secondary Sensory Cell Organ in Ascidians: In Search of the Ancestor of the Vertebrate Lateral Line PAOLO BURIGHEL,1* NANCY J. LANE,2 GASPARINI FABIO,1 TIOZZO STEFANO,1 GIOVANNA ZANIOLO,1 MARIA DANIELA CANDIA CARNEVALI,3 1 AND LUCIA MANNI 1 Dipartimento di Biologia, Università di Padova, I-35121 Padova, Italy 2 Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, United Kingdom 3 Dipartimento di Biologia, Università di Milano, I-20133 Milano, Italy ABSTRACT A new mechanoreceptor organ, the “coronal organ,” located in the oral siphon, is described by light and electron microscopy in the colonial ascidians Botryllus schlosseri and Botrylloides violaceus. It is composed of a line of sensory cells (hair cells), accompanied by supporting cells, that runs continuously along the margin of the velum and tentacles of the siphon. These hair cells resemble those of the vertebrate lateral line or, in general, the acoustico-lateralis system, because they bear a single cilium, located centrally or eccentrically to a hair bundle of numerous stereovilli. In contrast to other sensory cells of ascidians, the coronal hair cells are secondary sensory cells, since they lack axonal processes directed towards the cerebral ganglion. Moreover, at their base they form synapses with nerve fibers, most of which exhibit acetylcholinesterase activity. The absence of axonal extensions was confirmed by experiments with lipophilic dyes. Different kinds of synapses were recognized: usually, each hair cell forms a few afferent synapses with dendrites of neurons located in the ganglion; efferent synapses, both axo-somatic (between an axon coming from the ganglion and the hair cell) and axo-dendritic (between an axon coming from the ganglion and an afferent fiber) were occasionally found. The presence of secondary sensory cells in ascidians is discussed in relation to the evolution of sensory cells and placodes in vertebrates. It is proposed that the coronal organ in urochordates is homologous to the vertebrate acousticolateralis system. J. Comp. Neurol. 461:236 –249, 2003. © 2003 Wiley-Liss, Inc. Indexing terms: Urochordata; hair cells; tentacles; oral siphon; sensory system; placodes Ascidians (Urochordata) are filter-feeding invertebrates possessing a swimming tadpole larva which exhibits the structural body plan of chordates (Burighel and Cloney, 1997). The larval central nervous system (CNS) derives from a typical neural tube and shows a close relationship with the vertebrate nervous system, sharing with the latter similarities in organization and developmental patterning mechanisms (Wada et al. 1996, 1998; Shimeld and Holland, 2000; Manni et al., 2001). At metamorphosis, this nervous system degenerates and a remnant of the neural tube, the neurohypophyseal duct, produces the neural complex of the sessile adult (Burighel et al., 1998; Manni et al., 1999, 2001). This consists of an invertebratelike cerebral ganglion associated with a neural gland which, posteriorly, continues with a dorsal strand flanked by a plexus of neurons reacting positively to antibody for the hypothalamic gonadotropin-releasing hormone © 2003 WILEY-LISS, INC. (Mackie, 1995). The peripheral nervous system derives from nerves extending from the ganglion and from sensory cell bodies located at the periphery of the body; nerves are mixed, containing axons of both sensory and motor neu- Grant sponsor: Ministero della Università e Ricerca Scientifica e Tecnologica and University of Padova (P.B.); Grant sponsor: Emily Mary Pratt Musgrave Fund (N.J.L.). *Correspondence to: Paolo Burighel, Dipartimento di Biologia, Università di Padova, via U. Bassi 58/B, I-35121 Padova, Italy. E-mail: [email protected] Received 17 October 2002; Revised 21 January 2003; Accepted 1 February 2003 DOI 10.1002/cne.10666 Published online the week of May 5, 2003 in Wiley InterScience (www. interscience.wiley.com). SECONDARY SENSORY CELLS IN ASCIDIANS rons (Mackie and Wyeth, 2000; Burighel et al., 2001; Zaniolo et al., 2002). Ascidians possess a variety of receptors, whose sensory cells usually bear modified cilia. These are generally classified as mechano-, vibration-, gravity-, chemo-, photo-, and proprio-receptors (Bone and Mackie, 1982). As commonly occurs in invertebrates (Budelman, 1989), all these receptor cells are primary sensory cells, since they have their cell body located at the periphery and send their axon into the CNS. An exception to this pattern is seen in the appendicularians, planktonic animals considered to be the earliest lineage to diverge within the urochordates (Wada, 1998). Appendicularians possess unusual secondary sensory cells which form electrical synapses at their base with peripheral nerve fibers coming from the CNS (Bone and Ryan, 1979; Bone et al., 1979; Bollner et al., 1986; Bone and Mackie, 1982). Among the mechanoreceptors of tunicates, the cupular sense organs are of special interest. These are found in the atrium of the adult of the ascidian Ciona intestinalis (Fedele, 1923; Millar, 1953; Goodbody, 1974; Bone and Ryan, 1978) and salps (thaliacean urochordates) (Fedele, 1920). Their name derives from the presence of groups of ciliated cells embedded in a gelatinous cupula. Their importance derives from their similarities with the vertebrate otic and lateral line receptors. Unlike vertebrate hair cells, however, which are dedicated receptors that require secondary innervation from neurons located in the sensory ganglia of the VIIIth cranial nerve, the cupular sense organs are constituted of primary sensory neurons whose axons project directly to the CNS. Another sense organ, the capsular organ, analogous to the cupular organ, was recently described by Mackie and Singla (2003) in the atrium of Chelyosoma productum. Its sensory cells are primary receptors bearing a collar of microvilli surrounding a long cilium which projects into the capsule cavity. Cupular and capsular mechanoreceptors differentiate in the atrial chamber which receives the water coming from the pharynx through the gill slits. In botryllids, the atrial chamber arises as an ectodermal mid-dorsal anlage which extends ventrally to surround the pharynx at right and left (Manni et al. 2002). In more primitive ascidians, such as Ciona intestinalis, it originates from a pair of dorsal ectodermal invaginations which may be compared to the otic placodes of vertebrates, being topographically located in a corresponding position (Katz, 1983). The possible homology between the atrial primordia and the otic placodes was proposed by different authors on the basis of paleontological evidence (Jefferies, 2001), the anatomical relationships of the atrial primordia, the morphology of the cupular organs, and data coming from molecular biology, in that both the developing atria and otic placodes express members of the Pax2/5/8 gene family (Baker and Bronner-Fraser, 1997; Wada et al., 1998; Shimeld and Holland, 2000). In vertebrates, sensory cells are derived, together with the sensory neurons, from neural crest or placodes, traditionally thought to be unique to vertebrates (Northcutt and Gans, 1983). In particular, neural crest-derived receptors belong to primary sensory neurons, while placodederived receptors may be primary neurons (the olfactory sensory cells) or secondary sensory cells (the hair cells) associated with neurons. The hair cells are ciliary, highly differentiated mechanoreceptors and their name derives from the peculiar microvilli, called stereovilli (synony- 237 mous with stereocilia), that protrude into the fluid-filled cavities of the inner ear or lateral line organs. Secondary sensory cells are considered to be phylogenetically derived from primary sensory cells (Baker and Bonner-Fraser, 1997; Fritzsch and Beisel, 2001). The possibility that precursors of placodal cells may occur in modern invertebrate chordates, cephalochordates and urochordates, is of interest in relation to ideas concerning the evolutionary origin of vertebrate sensory cell types. In the cephalochordate Amphioxus, considered to be the closest living relative of vertebrates, different sensory cells, type I cells and type II cells, are found scattered in the rostral epithelium. Type I cells are primary sensory cells, while type II cells are secondary sensory cells, with synaptic terminals borne out on short extensions of the cell body (Bone and Best, 1978; Lacalli and Hou, 1999; Holland and Holland 2001; Holland and Yu, 2002). For both types of cells, neither their embryological origin nor their functional properties are known, although they are generally assumed to be either mechano- or chemoreceptors on the basis of their morphology. On the oral spines which project across the mouth there are secondary mechanoreceptor cells, the oral spine cells, involved in the rejection response (Lacalli et al., 1999). Despite differences in function, these sensory cells were considered homologous to the vertebrate taste buds which, on the other hand, are neither placodally nor neural crest-derived (Barlow and Northcutt, 1995). Thus, the available data do not resolve the evolutionary issue of how amphioxus sensory cells relate to the receptor cells of vertebrates. The possible homology between placodes (the hypothalamic-hypophyseal-olfactory placode) and an embryonic structure of ascidians (the neurohyphyseal duct) has recently been proposed (Manni et al., 1999, 2001). Moreover, in ascidians Burighel and Cloney (1997) have reported the presence of secondary sensory cells in the oral siphon of botryllids; such cells bear a short cilium with microvilli and their basal plasmalemma is in contact with a number of fine nerve fibers. The oral siphon of most ascidians bears, at its base, a crown of tentacles capable of responding to different kinds of stimuli (mechanical, thermal, chemical) (Hecht, 1918a, b; Day, 1919), but the presence of sensory cells on them has only been previously mentioned by Seeliger (1893). In the present study we examined, by scanning and transmission electron microscopy, the oral and atrial siphons in two ascidians, Botryllus schlosseri and Botrylloides violaceus, in order to verify unequivocally the presence of sensory cells. Here we describe a novel sensory organ, the coronal organ, located on the inner wall of the oral siphon. Its ciliated receptor cells are innervated by secondary sensory neurons, located in the cerebral ganglion, and aligned in such a way so as to form a continuous sensorial line, strongly reminiscent of the lateral line system of vertebrates. MATERIALS AND METHODS Specimens of Botryllus schlosseri and Botrylloides violaceus Oka, 1927 (family Styelidae, order Stolidobranchia) used in this study were from the laboratory, where colonies adhering to glass are routinely cultured according to Sabbadin’s (1955) technique. These colonies were originally collected from the lagoon of Venice. Each colony contains numerous blastozooids interconnected by a vas- 238 P. BURIGHEL ET AL. cular network running throughout the common tunic and grown through regular cycles of blastogenic generations. The two species differ greatly in the size and morphology of the larva, which, in Botrylloides violaceus, has a trunk about 1 mm in length with about 30 –32 ampullae and, in Botryllus schlosseri, a trunk of about 450 m, with eight ampullae. In both species the larva metamorphoses into a sessile oozooid, the founder of the colony, which grows through a series of blastogenic generations. The blastozooids of Botrylloides violaceus are arranged in doublerow systems parallel to one another, while the blastozooids of Botryllus schlosseri are grouped in star-shaped systems around the common cloacal siphon. Whole-mount preparation Colonies were anesthetized with MS 222, fixed in Bouin’s fluid, washed in 50% ethyl alcohol, rehydrated, and stained with Mayer’s hematoxylin. After washing in distilled water, they were dehydrated in alcohol and mounted with balsam. Transmission electron microscopy More than 30 adult blastozooids selected from different colonies were anesthetized with MS222 and fixed in 1.5% glutaraldehyde buffered with 0.2 M sodium cacodylate, pH 7.4, plus 1.6% NaCl. After washing in buffer and postfixation in 1% OsO4 in 0.2 M cacodylate buffer, the specimens were dehydrated and embedded in Araldite. Thick sections (1 m) were counterstained with toluidine blue; thin sections (60 nm) were given contrast by staining with uranyl acetate and lead citrate. Transverse serial thin sections of tentacles were cut and analyzed in order to verify the presence of axonal processes extending from the basal surface of the sensory cells. Micrographs were taken with a Hitachi H-600 electron microscope operated at 80 kV. To reveal acetylcholinesterase (AChE), tissue was treated following the method of Gautron (1982), prefixing the specimens in 1.5% glutaraldehyde buffered with 0.1 M sodium cacodylate, pH 7.4, containing 5% sucrose and using acetylthiocholine iodide (Sigma, St. Louis, MO) as substrate. Specimens were then postfixed in 1% OsO4 in phosphate-buffered-sucrose, dehydrated, and embedded in Araldite. Ultrathin sections were stained with uranyl acetate and lead citrate. Controls were treated as described above, but without the substrate. Scanning electron microscopy Pieces of colony containing adult blastozooids were fixed as described for transmission electron microscopy. After dehydration, dissection was performed in order to expose the oral siphon lumen with the velum and tentacles. Specimens were critical-point dried, sputter-coated with goldpalladium, and observed under a Cambridge Stereoscan 260. Axonal tracing technique In order to verify if sensory cells are primary or secondary, the lipophilic membrane dye 1,1⬘-dioctadecyl3,3,3⬘,3⬘-tetramethylindocarbocyanine perchlorate (DiI) (Aldrich, Milwaukee, WI) was applied on the surface of velum and tentacle cells using a capillary tube attached to a micromanipulator. The rationale was that, following an extracellular application, the DiI readily diffuses over the whole membrane of the cell, rather than remaining in and diffusing through the aqueous cytoplasmic component or extracellular spaces; thus, if the cells under consideration are primary neurons, applying the dye on the cell bodies makes it possible to follow the pathway of their entire axonal projections. In contrast, if the cells are secondary neurons then the dye marks as a spot on each cell (Clarke, 1999). A small aliquot of DiI (at the concentration of 3 mg/mL in DMF) was delivered in the interior of the oral siphon to touch the velum and surface of the tentacles. The dye deposit was checked with a conventional fluorescence microscope at the time of and after the application. DiI fluoresces an intense red. The dye in excess was gently removed putting the injected colonies into filtered seawater. All photos were acquired with a Duoscan (Agfa), had levels adjusted, were collated, and typeset in Corel Draw 9. RESULTS General organization of the oral and atrial siphons Each blastozooid of Botryllus schlosseri and Botrylloides violaceus, in every system of the colony, has its own oral (or inhalant) siphon which opens independently at the periphery. The atrial (or exhalant) siphon converges, together with the siphons of the other zooids, in a common central cloacal chamber, in the case of Botryllus schlosseri (Fig. 1A), or in a common, long cloacal channel, in the case of Botrylloides violaceus. Following Millar (1953) and Burighel and Cloney (1997), the “oral siphon” comprises the area between the external rim of the siphon and the ring of the velum from which the tentacles project (Fig. 1B–D). In the oral siphons of both species analyzed, the crown of tentacles is formed of first- and second-order tentacles. There are four first order tentacles symmetrically arranged: two long, lateral ones, located on the right–left axis, and two shorter, medial (ventral and dorsal) ones. The shorter, second-order tentacles alternate regularly with the first-order ones. In Botryllus schlosseri, Fig. 1. Botryllus schlosseri. A: One star-shaped system of a colony showing the oral siphons (arrows) of each blastozooid (b) and the central, common cloacal chamber (asterisk). t, tunic. Scale bar ⫽ 1.5 mm. B: Oral siphon of a blastozooid. dt, dorsal tentacle; lt, lateral tentacle; vt, ventral tentacle; arrowheads, second order tentacle. Whole-mount preparation. Scale bar ⫽ 150 m. C: Detail of a lateral tentacle. Whole-mount preparation. Scale bar ⫽ 60 m. D: Section of an oral siphon. The tunic (arrowheads) covers the inner side of the siphon and part of the velum (v). t, tentacles. Scale bar ⫽ 60 m. E: Oral siphon as seen from the inner side of the blastozooid. velum (v), and lateral (lt), ventral (vt) and dorsal (dt) tentacles. dl, dorsal lamina; pb, pericoronal band. Scanning electron microscopy. Scale bar ⫽ 90 m. F: Velum (v) and lateral tentacle (lt) seen from the outer side. Note the ciliated cells (arrowheads) of coronal organ. t, tunic. Scanning electron microscopy. Scale bar ⫽ 20 m. G: Ciliated sensory cells (arrows) of coronal organ on lateral tentacle. Arrowheads point to the line of microvillar protrusions (enlarged in the inset) of supporting cells. One asterisk, upper side of the tentacle; two asterisks, flank of the tentacle. Inset: detail to show the microvillar protrusions flanking the sensory cells. Scanning electron microscopy. Scale bar ⫽15 m; inset scale bar ⫽ 2 m. H,I: Hair cells on the velum. Each bears a short, stiff cilium (c) accompanied by a group of stereovilli (arrowheads) as visible in detail in the inset. In I, microvillar protrusions (arrows) of supporting cells are visible. Scanning electron microscopy. Scale bar ⫽ 3 m in H; scale bar ⫽ 2 m in inset; scale bar ⫽ 1 m in I. SECONDARY SENSORY CELLS IN ASCIDIANS 239 Figure 1 240 P. BURIGHEL ET AL. extends into the siphon as far as the atrial velum; the epidermis is continuous with the atrial epithelium. On the epidermis of the atrial siphons of both species, we did not find any sensory structure. In particular, there were no cupular or capsular organs like those described from the atrial siphon of Ciona intestinalis (Bone and Ryan, 1978) and Chelyosoma productum (Mackie and Singla, 2003), respectively. However, on the outer border of the tentacles and velum of the oral siphons of both species we found a row of previously undescribed sensory cells, which are completely exposed to the inhalant current of the seawater and are arranged in such a way as to constitute a sensory organ. This novel organ, which forms a continuous sensory line bordering the crown of velum and tentacles (Fig. 1F,G), will be referred to here as the coronal organ. We did not find any other sensory structures in the oral siphons of the two species examined. The histological organization of the oral siphon in Botryllus schlosseri and Botrylloides violaceus is similar — the data used here document the organization of the novel sensory organ was obtained mainly from observations on Botryllus schlosseri. Coronal organ Fig. 2. A: View of the inner side of the siphon to show the coronal organ bordering the anterior face of the velum and tentacles. The tunic covering the epidermis is not indicated. B: Sketch showing the zigzag arrangement of hair cells in the coronal organ. the lateral tentacles are ⬃80 m long, the dorsal and ventral ones are ⬃55 m long, and the second-order tentacles are ⬃33 m long (Fig. 1E,F). The epidermis extends onto the inner surface of the oral siphon and is continuous with the pharyngeal epithelium. The velum is small and circular and extends between, and is continuous with, the tentacles themselves. The tunic covers the epidermis on the inner side of the siphon and gets thinner in the proximal area of the tentacles and the velum (Fig. 1D). In normal conditions, when the tentacles are raised, they are extended on the same plane of the velum and form a sort of filter at the base of the siphon (Fig. 1B). In relaxed conditions the tentacles bend down toward the branchial chamber (Fig. 1D). As they lack their own musculature, their movements are due to a combination of different factors, such as the pressure of blood and the activity of siphon muscles. We define “atrial siphon” in Botryllus schlosseri and Botrylloides violaceus as the region between the rim of the atrial siphon and the atrial velum, which marks the outer border of the atrial chamber. It is noteworthy that the atrial siphon, although somewhat different in the two species, in both cases extends an epidermal languet on its dorsal side which comes together, with the languets of adjacent zooids, to form the roof of the common cloacal chamber/channel. Like the oral siphon, in the atrial siphon the epidermis is accompanied by the tunic, which When observed under the scanning electron microscope (Fig. 1E,F), the tentacles and the velum of the oral siphon exhibit a smooth surface and no particular specialization of cell apexes can be recognizable, except for the sensory cell line of the coronal organ (Figs. 1G–I, 2). We called these sensory cells “hair cells” because each one is characterized by tufts of stereovilli and a single cilium. The cilium is about 4 m in length and 280 nm wide; stereovilli are long and rod-like, 2–2.4 m in length and about 110 nm in diameter. The hair cells have a zigzag arrangement in a row bordering the apex of the velum and the upper side (facing the external siphonal rim) of tentacles (Fig. 2B). More precisely, the file of hair cells runs along the margin of the velum and the upper side of each tentacle in Fig. 3. Botryllus schlosseri. Transmission electron microscopy. A: Longitudinal section of the dorsal tentacle showing the tunic (t) partially covering it. Numerous blood cells (bc) are inside the tentacle. Scale bar ⫽ 8 m. B: Transverse section of a tentacle. Hair cells (hc) are located at the border of anterior side (top) of the tentacle. Some nerve fibers (arrowheads) are visible adjacent to the basal plasmalemmata of hair cells. sc, supporting cell. Scale bar ⫽ 4 m. C: A hair cell (hc) accompanied by the supporting cells (sc). Hair cell possesses a basal nucleus (n), rough endoplasmic reticulum cisternae (rer), a supranuclear Golgi apparatus (G), and a hair bundle composed of a cilium (c) accompanied by numerous stereovilli (s). The apical plasmalemma of supporting cells present a thick glycocalyx (arrowheads) and microvillar protrusions (arrows). bl, basal lamina; cr, ciliary rootlets; mb, multivesicular body; nf, nerve fibers. Scale bar ⫽ 2.2 m. D: Detail of the apical area of a hair cell. Note that the cell presents an eccentric cilium (c), as demonstrated by its insertion close to the lateral plasmamembrane. Arrow, centriole; arrowheads, ciliary rootlets; bb, basal body of the cilium; G, Golgi apparatus; m, mitochondrion; s, stereocilium; sc, supporting cells; tj, tight junction; za, zonula adherens. Scale bar ⫽ 0.3 m. E: Longitudinal section of hair cells. Note the cylindrical shape of the cells. Arrowheads, sensory cilia; s, stereovilli; n, nucleus. Scale bar ⫽ 2 m. F,G: Tangential sections of the hair bundle showing the zigzag arrangement of hair cells, some of which bear an eccentric cilium (arrows). The cilium (c) is accompanied by 90 –95 stereovilli (s). G: Enlargement of F and shows in detail in inset the fibrils (arrowheads) extending radially between the cilium and the adjacent stereovilli. Scale bar ⫽ 2 m in F; 0.35 m in G; 0.14 m in inset. SECONDARY SENSORY CELLS IN ASCIDIANS 241 Figure 3 242 P. BURIGHEL ET AL. such a way as to form a continuous ring at the base of the siphon, exposed to the inhalant seawater flow (Fig. 2A). In a lateral tentacle of Botryllus schlosseri it is possible to find about 150 –200 hair cells and the whole organ is constituted of about 2,000 hair cells. In sections (Fig. 3A,B), the epithelium of both the velum and the tentacles is generally flat and becomes thicker at the level of hair cells. Each tentacle contains nerve fibers (we were able to count 15–20 neurites in sections of the proximal part of the lateral tentacles), often located at the base of hair cells. Blood cells were also present, but not muscle cells, although the latter are present in the mantle at the base of the tentacles. Supporting cells The file of sensory cells is flanked on both sides by supporting cells whose lateral surface contacts the hair cells (Fig. 3C). Supporting cells are similar to adjacent epithelial cells in cytoplasmic characteristics, but differ in position and shape. They are C-shaped cells and actually enclose the sensory cells, so as to isolate them. Apically, supporting cells elongate until they reach the collar of stereovilli; their lateral membrane follows the lateral membrane of the hair cells; basally, they partially cover the sensory cells reducing their area available to contact the nerve fibers. Apically, in proximity to the sensory cells, they raise to form small, aligned microvillar protrusions which, in their whole, delimit a sort of canal in which the sensorial cilia and stereovilli are found (Figs. 1G,I, 2B, 3C). The supporting cells possess an apical, thick, and very dense glycocalyx, which is also present on contiguous epithelial cells but not hair cells. All the cells of the velum and tentacles, both sensory and nonsensory, are joined apico-laterally to each other by dense, tight junctions; often, below them a zonula adherens can be recognized (Fig. 3D). Sensory cells The sensory cells are cylindrical (Fig. 3E) and are joined to the adjacent cells by tight junctions, whereas gap junctions were never observed on their lateral surfaces. Sensory cells have a large basal nucleus and scattered mitochondria and RER cisternae. The Golgi complex lies above the nucleus and is composed of a few stacks of cisternae and associated vesicles (Fig. 3C). The hair cells bear an apical structure (Fig. 3D–G) composed of a stiff cilium accompanied by about 90 –95 long stereovilli. The position of the cilium varies: it may be found central to the corolla of stereovilli, or may be completely eccentric, where it inserts with its basal body close to the lateral cell membrane. In the latter case, the tuft of stereovilli occupies the remainder of the apical membrane. The distribution of the two subtypes of hair cells (with centric or eccentric cilium) appears random in the coronal organ. Each cilium has a conventional 9⫹2 microtubular arrangement, with a dense, short basal body connected to poorly developed ciliary rootlets; a secondary centriole is sometimes recognizable, lying perpendicularly to the first (Fig. 3D). The stereovilli are stiff, unbranched, all the same length, and are filled with microfilaments that are continuous with the cytoskeletal filaments of the apical cytoplasm. Both the ciliary and stereovilli membranes are covered by a fibrillar fuzzy coat (Fig. 3G, inset). Several of the coat fibrils, up to 40 nm long, extend radially to estab- lish connections between adjacent stereovilli and between the stereovilli and the ciliary shaft. Synaptic connectivity The basal plasmalemma of each hair cell lies on a basal lamina, which forms a continuous fibrous layer supporting both sensory and the other epithelial cells (Fig. 3C). At certain sites the basal plasmalemma of sensory cells exhibits infoldings in the form of one or two grooves, which are also bounded by extensions of supporting cells (Fig. 4A). In these grooves the typical extracellular matrix is absent (Figs. 4A–D, 5). The grooves contain numerous neurites showing a strict relationship with the sensory cell membrane. The basal lamina of the sensory cells intermingles with the fibrous matrix enveloping the nerve fibers. A few nerve fibers are also grouped in the central blood sinus of the tentacles and the velum. Frequently, in longitudinal sections of sensory cells a small group of nerve fibers can be seen to pass from one hair cell to the adjacent ones running inside their basal groove; we occasionally found branching fibers or neurites entering the basal groove coming from the central nerve fibers. A number of conventional axo-somatic synapses were seen on the sensory cells and axo-axonic synapses were also recognized (Figs. 4, 5) in the vicinity of the sensory cells. Synaptic contacts were identified by paired, thickened, electron-dense plasma membranes lying in parallel 20 –25 nm apart, with regularly arranged filaments traversing this cleft and small vesicles attached to the membrane dense material on one or both sides of the cleft. The dense material consisted of an amorphous layer close to the cytoplasmic membrane face, being more pronounced on the postsynaptic side. This configuration corresponds to the synapses described in the cerebral ganglion of Botryllus schlosseri (Burighel et al., 1998; Manni et al., 1999). In some cases the same presynaptic area, furnished with numerous vesicles, was seen to make synaptic contacts with several different neurites, reaching a width of 1.3 m as in the case of the synapses shown in Figure 4A. Besides the unidirectional synapses, which were predominant, synapses were found that had vesicles on both sides. Within the active zone we could distinguish synaptic vesicles with a homogeneous, moderately electron-dense content, or synaptic vesicles containing a dense core surrounded by a pale halo (Fig. 4C). The system of neurite-soma connections was mainly represented by afferent synapses (Figs. 4A–C), recognizable in that the presynaptic membrane, associated with the synaptic vesicles, belongs to the hair cell. These synapses are involved in transferring the sensorial information from the hair cells to a dendrite of a neuron, whose soma is in the cerebral ganglion. Frequently we observed the same hair cell to form 2–3 afferent synapses with different neurites. Possible efferent synapses were occasionally recognized. In these, the presynaptic membrane belongs to an axon of a neuron whose soma is located in the cerebral ganglion, while the postsynaptic membrane belongs to a hair cell (Fig. 4D). Moreover, we observed axo-dendritic synapses (Figs. 4D, 5) in the basal groove created by hair cells, or outside the groove, at the base of sensory cells, as well as synapses between neurites and supporting cells (Figs. 4A, 5). Sections treated to reveal the enzyme AChE activity showed a reaction product, in the form of minute, electron- SECONDARY SENSORY CELLS IN ASCIDIANS 243 Fig. 4. Transmission electron microscopy of synaptic connectivity of the coronal organ of Botryllus schlosseri. A: Numerous nerve fibers (nf) are located in grooves formed by the basal plasmalemma of the hair cell (hc) and the supporting cell (sc) and delimited by the basal lamina (bl). On the left, an extended synaptic area, marked by numerous presynaptic vesicles (arrowheads) close to the hair cell plasmalemma is visible. Arrow, synapses between a nerve fiber and a supporting cell; n, nucleus. Scale bar ⫽ 0.3 m. B,C: Afferent synapsis (white arrows) at the base of two adjacent hair cells (hc). The afferent synapses are recognizable for the presence of presynaptic vesicles close to the basal plasmalemma of hair cell and a pronounced density on the membrane of the dendrite (d). C: An enlargement of the squared area of B, close to the hair cell plasmalemma, presynaptic vesicles with homogeneous, moderately electron-dense content (black arrow) and synaptic vesicles containing a dense core surrounded by a pale halo (white arrowhead). bl, basal lamina; m, mitochondrion in dendrite; nf, nerve fiber. Scale bar ⫽ 0.4 m in B; 0.15 m in C. D: Efferent axo-somatic synapsis (white arrows): the presynaptic membrane belongs to the axon (a), while the postsynaptic membrane belongs to the hair cell (hc), as shown by the density of the plasmamembrane. An axo-dendritic synapsis (arrowheads) is also visible in tangential section. bl, basal lamina; nf, nerve fiber. Scale bar ⫽ 0.25 m. dense granules, which marked the profile of nerve fibers and was concentrated either in their membrane close to sensory cells, or was free in the narrow, basal intercellular spaces between adjacent sensory cells (Fig. 6A, B). The product was never found in the cytoplasm of hair cells. Although the basal membrane of hair cells often elongate to form protrusions that partially embrace the nerve fibers, long cellular processes, recalling axonal fibers typical of primary sensory cells, were never encountered. In this respect, when applied in vivo at the base of the oral siphon, the lipophilic dye DiI marked the coronal organ cells but never revealed the presence of cellular projections arising from sensory cells and reaching the cerebral ganglion (Fig. 6C). The main characteristics of sensory cells are schematically represented in Figure 7. DISCUSSION Sensory organs in urochordates Our data demonstrate the presence of a novel sensory organ, the coronal organ, in the oral siphon of Botryllus schlosseri and Botrylloides violaceus, formed from specialized cells located in the upper side of the velum and tentacles exposed to the inhalant seawater flow. These sensory receptor cells are flanked by supporting cells and bear apically a cilium accompanied by numerous, long, 244 Fig. 5. Schematic drawing of synaptic connectivity between the basal plasmalemma of a hair cell and nerve fibers. One synapsis, whose direction is not indicated, is between a supporting cell and a nerve fiber. stiffened stereovilli, while basally they form invaginations into which nerve fibers run. Numerous unequivocal synapses were found between the basal membrane of these sensory cells and the underlying nerve fibers, proving that these receptor cells are secondary sensory cells. The presence of possible sensory cells in the tentacles of ascidians was mentioned by Seeliger (1893) in the species Ciona intestinalis, but ignored by subsequent authors. More recently, Burighel and Cloney (1997) suggested, in the tentacles of the oral siphon of Botryllus schlosseri, presumptive secondary sensory cells, a report now confirmed by our data. Urochordates possess a variety of receptor systems for different kinds of stimuli (Bone and Mackie, 1982; Bone, 1998; Mackie and Singla, 2003). As generally occurs in invertebrates (Budelmann, 1989), receptor cells are simple primary sensory cells, scattered or grouped, usually bearing cilia that are modified in some way. An exception is found in the appendicularians, where particular secondary sensory cells form electrical synapses (gap junctions) with neurites derived from the central nervous system (Bone and Ryan, 1979; Bollner et al., 1986; Bone, 1998). However, this seems to represent an unusual modification that, together with other features, both morphological (Fenaux, 1998; Burighel et al., 2001) and genomic (Wada, 1998), render the appendicularians distinct from the other urochordates. Features and function of the coronal organ The morphology of the sensory coronal cells provides some information regarding coronal organ function, suggesting that it might be a hydrodynamic receptor system, i.e., a responsive mechanoreceptor system sensitive to any kind of motion in the water surrounding it or to particles in its aquatic environment (Budelmann, 1989). The ascidian larva has both a well-developed photoreceptor and a balance organ as navigational aids for exploring the environment and selecting the best substrate on which to fix and metamorphose (Burighel and Cloney, P. BURIGHEL ET AL. 1997; Sorrentino et al., 2000; Lacalli, 2001); in contrast, the adult has a sessile mode of life and relatively little demand is placed on its sensory system (Goodbody, 1974). Almost all the adult’s sensory organs are concerned with protection or defense and, among them, hydrodynamic receptors play an important role in behavioral reactions to water movements, which occur during feeding or in response to touch. It was previously supposed that the atrial cupular organ of Ciona intestinalis (Bone and Ryan, 1978) and the capsular organ of Chelyosoma productum (Mackie and Singla, 2003) were mechanoreceptors involved in monitoring and regulating the water current through the atrial cavity. In Botryllus schlosseri and Botrylloides violaceus, we did not find sense organs in the atrial siphons; nevertheless, animals of both species are able to respond to vibrations, both transmitted through the solid base of their attachment and from the seawater (unpubl. data). In the absence of cupular sense organs, the hydrodynamic receptors, if present, must be located elsewhere. Indeed, botryllid zooids do not have their atrial siphon opening directly to the exterior, as occurs in solitary species such as Ciona intestinalis and Chelyosoma productum, but into a common cloacal chamber/channel. A more logical location for hydrodynamic receptors might therefore be in the oral siphon, rather than the atrial siphon. Physiological studies on solitary ascidians demonstrated that the tentacles are sensitive to mechanical and chemical stimuli and are a useful device in filtering out any large particles from the inhalant water before they reach the pharynx (Hecht, 1918a, b; Day, 1919). For its location and its morphological features, the coronal organ represents a good candidate for a hydrodynamic receptor system in botryllids, having receptor cells exposed to the inhalant current and bearing a cilium accompanied by stereovilli. In most invertebrate phyla, epithelial mechanoreceptor cells sensitive to water movements carry a concentric hair bundle of a single cilium surrounded by a stiff cylinder of interconnected stereovilli (collar configuration). This configuration is already known to exist in cnidarian polyps and medusae and is also the standard for the epithelial mechanoreceptors in the lower chordates (Budelmann, 1989, 1992; Thurm et al., 1998). In this respect, it is equivalent to the lateral line receptors of fish and amphibians, although these latter have an eccentric configuration of the cilium and stereovilli arranged in diminishing order. In botryllids, the cilium of hair cells was found either to be central to the corolla of stereovilli, as normally occurs in ascidian sensory structures, or to be completely eccentric. This is of particular interest, since the collar configuration is considered to be the predecessor of the eccentric hair bundle found in the otic and lateral line receptors of vertebrates (Thurm et al., 1998). In sensory coronal cells the mechanoreceptor role is also supported by the features of the hair bundle. We observed that, although the cilium has a typical internal 9⫹2 configuration and a pair of centrioles at its base, it is short, relative, for example, to stigmatal cilia (Martinucci et al., 1992); it has short ciliary roots and appears stiff in that it was never found bent. These features indicate that it is not capable of active beating, so it cannot be involved in generating current flow or moving particles. On the other hand, the hair cells do not have the features of chemoreceptors because the latter usually have long (sometimes branched) microvilli and lengthy cilia embedded within the mucous secretion of accessory gland cells (Altner and SECONDARY SENSORY CELLS IN ASCIDIANS 245 Prillinger, 1980; Finger, 1997). The supporting coronal cells do not show any particular signs of secretory activity at the apex, where they have a thick apical glycocalyx and microvillar protrusions arranged to create a sort of channel for collecting the hair bundles of the sensory cells. Moreover, the cilium is connected to the surrounding stereovilli by extracellular radial filaments. It is possible that the fibrillar links represent the structural device for transduction of the stimulatory force acting on the cilium to the site of mechanosensitive ion channels, as demonstrated in other mechanoreceptor systems (Gillespie, 1995; Thurm et al., 1998). The presence of numerous and well-developed organelles involved in protein synthetic processes, such as numerous mitochondria, well-developed rough endoplasmic reticulum, and Golgi apparatus, suggests that sensory cells are very active, possibly involved in the synthesis of neurotransmitters. Hair cells possess tight junctions which help in maintaining distinction of the apical from the basolateral plasmalemmata domains. However, in comparison with other ascidian epithelia (Lane et al., 1986) they have no gap junctions, the absence of which indicates that the sensory cells are metabolically and electrically isolated from the adjacent ones. All the above suggests that the coronal organ could both detect the impact of small particles and/or respond to vibration; in the latter role, the velum might act like a sort of a basilar membrane, vibrating in response to waterborne vibrations in the near field and transmitting these movements to the hair cells. Structure of the synaptic contacts Neurites run in grooves at the base of sensory cells, establishing synaptic contacts of various categories. The basal lamina, delimiting these grooves, isolates neurites from the blood compartment and favors the strict spatial relation between them and the sensory cells. The evidence of synapses was critical in order to classify the hair cells as secondary receptors. This conclusion was corroborated by the absence of axonal processes emanating from the receptors directed towards the cerebral ganglion. Although we analyzed many specimens, including some serially sectioned for observation with the electron microscope, none were found. Moreover, the experiments performed in vivo with the lipophilic dye DiI on Botryllus schlosseri confirmed that hair cells are secondary receptors. This dye is often used to visualize the pathway of neurites (Clarke, 1999). When applied to the neuronal soma, it spreads along its protrusions permitting their tracing; vice versa, when applied to the peripheral nerve fibers it permits the localization of their soma. Our experiments demonstrated that a discontinuity existed between each sensory cell and the underlying nerve fibers because Fig. 6. Botryllus schlosseri. A,B: Specimens treated to reveal AChE reaction product. Electron-dense granules (arrowheads) are located at the base of hair cells (hc) on the membranes of nerve fibers (nf) and in intercellular spaces (white arrowhead). B: Enlargement of the squared area of A. bc, blood cell; c, cilium; s, stereocilium; sc, supporting cell. Transmission electron microcopy. Scale bar ⫽ 1.5 m in A; 0.5 m in B. C: The DiI marks the hair cells on the velum (v) and tentacles (t). No neurites can be seen arising from hair cells and projecting toward the cerebral ganglion. Fluorescence microscopy. Scale bar ⫽ 70 m. 246 P. BURIGHEL ET AL. Fig. 7. Schematic drawing of a hair cell accompanied by supporting cells. no axons were marked when the DiI was applied inside the siphon. It is also of note that, probably because of the presence of the thick glycocalyx and tunic, the dye only marked the sensory cells of the coronal organs, while other cells of the tentacles and velum and cells of the siphonal epithelium did not become stained. Hair coronal cells are contacted by two principal sets of neurons, both with their nucleus located in the cerebral ganglion: the afferent neurons, transmitting information from the hair cells to the ganglion, and the efferent neurons, providing input to the hair cell and/or sometimes to the hair cells’ afferents. This situation is reminiscent of what is known for the hair cells of the inner ear and the lateral line of vertebrates. Where synapses were recognized it was possible to classify the fibers as afferent (dendritic) and efferent (axonal) ones: afferent synapses were mainly found, although axo-somatic and axodendritic efferent synapses were also encountered. In ascidians, synapses between peripheral fibers were never recognized. However, in consideration that experimentally deganglionated animals maintain a neural net able to guarantee responses to tactile and electrical stimulation, Mackie and Wyeth (2000) hypothesized the presence of synaptic interconnections in its periphery. Our data show that synapses occur between peripheral fibers and thus provide a possible explanation for how deganglionated animals may regulate their activities. AChE histochemically labeled nerve fibers occur in the basal grooves of sensory coronal cells; its reaction product, analogous to the situation in the neuromuscular junctions of ascidians (Burighel et al., 2001), selectively marked the plasmalemmata and the intercellular spaces in these sites. This enzyme is known to label the vertebrate octavolateralis efferent neurons (Hellmann and Fritzsch, 1996), which, on the basis of the spatial and developmental associations with facial motor neurons, as well as their cholinergic nature, are thought to be rerouted motor neurons (Manley and Koppl, 1998). The AChE method had been successfully used in ascidians for revealing the motor system (Arkett et al., 1989) and positive nerve fibers were described in the tentacles of Botryllus schlosseri (Burighel et al., 2001; Zaniolo et al., 2002). If such nerves could play an efferent role the parallelism with the efferent system of vertebrates would be very striking. Thus, the morphological diversity and pattern of innervation of hair cells suggests the possibility of a different role for the two sets of coronal mechanosensory cells (with centric and eccentric cilium) and the possibility of fine control (as modulation or amplification of the mechanical signal) from the brain, as occurs in vertebrates (Hudspeth, 1997; Manley and Koppl, 1998; Fay and Popper, 2000; Smotherman and Narins, 2000). In Botryllus schlosseri we estimated that about 150 –200 hair cells and no more than 20 nerve fibers exist for each SECONDARY SENSORY CELLS IN ASCIDIANS lateral tentacle, and each hair cell forms 2–3 afferent synapses and at least one efferent synapse. This means that each nerve fiber has to form dozens of synapses in order to ensure that the innervation reaches all the sensory cells. Then, considering that the whole coronal organ contains about 2,000 hair cells, and that in the cerebral ganglion there are no more than 1,000 neurons (unpubl. data), and that branched nerve fibers underlying the sensory cells were rarely encountered, we hypothesize that a single dendrite, or axon, has to contact many different hair cells en passant. In many animals the complement of receptor cells far exceeds the number of central neurons that process sensory information (Derby and Steullet, 2001); in particular, the high degree of convergence of afferent fibers and divergence of efferent fibers found in the coronal organ shows similarities with the common lateral line and inner ear efferent system of Xenopus laevis (Hellmann and Fritzsch, 1996). It is also a remarkable feature of the coronal organ that some neurites synapse with the supporting cells. A similar situation was described in birds (Takasaka and Smith, 1971; Koppl, 2001). A particular case is in the secondary receptors (Langherans cells) of appendicularian oikopleurids (Bone and Ryan, 1979), in which the axon passes to the receptor cell synapses (via gap junctions) with the adjacent accessory cells and so receive input from two entirely different sources. In all the above-mentioned cases, comprising the botryllids, the meaning of synaptic contacts with the supporting cells have not been definitely clarified, but it is possible that the supporting cells play a role in the control of sensory cell activity. Coronal organ and the vertebrate acoustico-lateralis system As regards its morphological features, the coronal organ can be compared with the ear and lateral line organ of vertebrates (Bone et al., 1995). Both the coronal and the lateral line organs have mechanoreceptors: 1) in linear and symmetrical structures; 2) exposed to the seawater current; 3) located in a channel (in botryllids delimited by microvillar extensions of supporting cells); 4) possessing a hair bundle composed of straight stereovilli accompanying an eccentric cilium; 5) with a nonmotile cilium; 6) made up of secondary sensory cells; and 7) in relation with both afferent and efferent fibers, comprising a cholinergic system. There are, of course, differences between the two organs, notably because the coronal organ also possesses sensory cells with a centric cilium and lacks both stereovilli disposed in diminishing arrays and a gelatinous cupula secreted by the supporting cells. A gelatinous cupula is instead present in the cupular organ of Ciona intestinalis that Bone and Ryan (1978) proposed as a suitable structural starting point for the evolution of the acoustico-lateralis system of vertebrates. Cupular organs differentiate in the atrium, a chamber which develops from a pair of dorsal ectodermal invaginations which were present in the fossil mitrates considered to be the ancestors to extant chordates (Jefferies, 2001; Dominguez et al. 2002). Atrial invaginations are comparable to the otic placodes of vertebrates because both have a similar position in the embryo, are formed by ectodermal tissue, and express members of the Pax-2/5/8 gene family (Bone and Ryan, 1978; Katz, 1983; Wada et al., 1998). Nevertheless, as a marked difference, cupular hair cells are primary 247 sensory cells, whereas vertebrate hair cells are secondary sensory cells; on this basis, Mackie and Singla (2003) produce considerable doubts that hair cells of vertebrates and those of the cupular organ are homologous. As regards the genetic data, some authors (Kozmik et al., 1999; Holland and Holland, 2001) considered the possibility that the expression of Pax 2/5/8 gene has a role in formation of the gill slits, which occurs through perforation of both pharyngeal and atrial epithelia (Manni et al., 2002). On the other hand, other authors (Wada et al., 1998) interpret the expression of Pax 2/5/8 to support the homology between ascidian atria and otic placodes. It is noteworthy that in Halocynthia roretzi (a species of the same family of botryllids), Pax2/5/8 gene expression was found at the level of atrial primordia, but also in the primordium of the “pharynx,” considered to be homologous to the vertebrate stomodeum (Wada et al., 1998). These authors hypothesized that, in ascidians, this gene has been coopted into pharyngeal development, perhaps in connection with the evolution of similar cellular properties in the pharynx and atria. In addition, recent data on gene expression in early amphibian and chick embryos demonstrate that the potential to form otic derivatives is not exclusive to the ectodermal region close to the hindbrain, but that the anterior epiblast is also competent to form an otic placode, if taken at a sufficiently early age (Gallagher et al., 1996; Groves and Bronner-Fraser, 2000). Since the oral siphon, and then the coronal organ, derive from the anterior ectoderm of the embryo (Manni et al., 1999), all these genetic data suggest that the ancestor of chordates had a broad capacity to form otic derivatives, in particular, secondary mechanoreceptor cells. This ability would now be restricted to the posterior epiblast of the head in vertebrates, while it is also maintained in the anterior ectoderm in urochordates. Thus, the morphology, embryological origins, and developmental gene expression all support our idea that the coronal organ and the acousticolateralis system are homologous. This idea would be consistent also with the interpretation of Jefferies (2001) that the possible ancestors of chordates, the fossil calcichordates, possessed the precursor of the acoustico-lateralis system of extant craniates. The cephalochordates lack otic vesicles, possibly present in their mitrate ancestors (Jefferies, 2001). Secondary sensory cells have been described in amphioxus (Bone and Best, 1978; Lacalli and Hou, 1999; Lacalli et al., 1999). They are scattered in the rostral epithelium of the juvenile and classified, on the basis of their architecture, as probable chemoreceptors. Moreover, other secondary sensory cells (oral spine cells) are in the spines of the mouth of the amphioxus larvae (Lacalli et al., 1999). Although they are mechanoreceptors bearing a cilium and in synaptic contact with peripheral neurons, as vertebrate hair cells are, they are grouped in clusters and do not possess a hair bundle of stereovilli. Moreover, Lacalli et al. (1999) proposed that they are homologous to the vertebrate taste bud cells which are not of placodal origin. Thus, at the moment, available data on amphioxus are not able to contribute to the discussion of the evolution of secondary hair cells and the acoustico-lateralis system of vertebrates. Among invertebrates the cephalopods possess sophisticated movement receptor systems with ciliated sensory cells, which are reminiscent of those of vertebrates: these include an angular acceleration receptor system composed 248 P. BURIGHEL ET AL. of primary and secondary sensory hair cells and a system analogous to the vertebrate lateral line formed by primary sensory cells (Budelmann and Bleckmann, 1988; Budelmann, 1989, 1996). These sensorial systems display a high degree of similarity with the vertebrate inner ear; however, they represent a convergent solution that serves the same function and are certainly not homologous to the vertebrate sensory system (Budelmann, 1992). Since vertebrate secondary sensory cells develop from placodes, any evolutionary hypothesis about sensory cells in chordates has to consider the possibility that placodal structures were present in the chordate ancestor. This issue is therefore a subject for debates and the idea that placodes are not exclusive to vertebrates is currently being taken into consideration (Baker and Bronner-Fraser, 1997; Burighel et al., 1998; Wada et al., 1998; Manni et al., 1999, 2001; Graham, 2000; Shimeld and Holland, 2000; Begbie and Graham, 2001). Baker and Bronner-Fraser (1997) suggested a possible scenario about the lineage relationships of the primary and secondary cells in vertebrates. They assumed that the ancestral ciliary receptors were concentrated in patches on the head; later, some cells formed specialized receptor cells that lacked central projections, while others formed the neurons that innervated them (as we can see in the acoustico-lateralis system); finally, the receptors might have been lost from certain placodes but the sensory neurons retained and coopted into the innervation of nonplacodal receptors. This hypothesis is supported by data on the development of mechanosensors considered to be, from a genetic point of view, homologous across phyla (Fritzsch and Beisel, 2001). Considering the above data, we believe that the results of the present work represent additional evidence in favor of the hypothesis that the ancestor of chordates had the ability to differentiate secondary sensory cells and that the coronal organ of urochordates is homologous to the acoustico-lateralis, or lateral line, system of vertebrates. Thus, a more complete scenario can be envisaged regarding the origin of placodes in chordates in that the chordate ancestors not only possessed the olfactory and adenohypophyseal placodes maintained in the extant tunicates (Burighel et al., 1998; Manni et al., 1999), cephalochordates (see Holland and Holland, 2001), and vertebrates, but also possessed the placodes of the otic area and the genetic and cytological machinery to differentiate the acoustic-lateralis system, including the secondary receptor hair cells which are today present in both vertebrates and ascidians. ACKNOWLEDGMENTS We thank Mr. C. Friso for the drawings. LITERATURE CITED Altner H, Prillinger L. 1980. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int Rev Cytol 67:69 –139. Arkett SA, Mackie GO, Singla CL. 1989. Neuronal organisation of the ascidian (Urochordata) branchial basket revealed by cholinesterase activity. Cell Tissue Res 257:285–294. Baker CVH, Bronner-Fraser M. 1997. The origins of the neural crest. II. An evolutionary perspective. Mech Dev 69:13–29. Barlow LA, Northcutt RG. 1995. Embryonic origin of amphibian taste buds. Dev Biol 169:273–285. Begbie J, Graham A. 2001. The ectodermal placodes: a disfunctional family. Philos Trans R Soc Lond B 356:1655–1660. Bollner T, Holmberg K, Olsson RA. 1986. Rostral sensory mechanism in Oikopleura dioica (Appendicularia). Acta Zool (Stockh) 67:235–241. Bone Q. 1998. Nervous system, sense organs, and excitable epithelia. In: Bone Q, editor. The biology of pelagic tunicates. Oxford: Oxford University Press. p 55– 80. Bone Q, Best ACG. 1978. Ciliated sensory cells in amphioxus (Branchiostoma). J Mar Biol Assoc UK 58:479 – 486. Bone Q, Mackie GO. 1982. Urochordata. In: Shelton GAB, editor. Electrical conduction and behaviour in “simple” invertebrates. Oxford: Clarendon Press. p 473–535. Bone Q, Ryan KP. 1978. Cupular sense organs in Ciona (Tunicata: Ascidiacea). J Zool Lond 186:417– 429. Bone Q, Ryan KP. 1979. The langerhans receptor of Oikopleura (Tunicata: Larvacea). J Mar Biol Assoc UK 59:69 –75. Bone Q, Gorski G, Pulsford LA. 1979. On the structure and behaviour of Fritillaria (Tunicata: Larvacea). J Mar Biol Assoc UK 59:399 – 411. Bone Q, Marshall NB, Blaxter JHS. 1995. Sensory systems and communication. In: Bone Q, Marshall NB, Blaxter JHS, editors. Biology of fishes. Glasgow: Blackie Academic & Professional. p 219 –262. Budelmann BU. 1989. Hydrodynamic receptor systems in invertebrates. In: Coombs S, Görner P, Münz H, editors. The mechanosensory lateral line: neurobiology and evolution. New York: Springer. p 607– 631. Budelmann BU. 1992. Hearing in nonarthropod invertebrates. In: Webster DB, Fay RR, Popper AN, editors. The evolutionary biology of hearing. New York: Springer. p 141–155. Budelmann BU. 1996. Active marine predators: the sensory world of cephalopods. Mar Fresh Behav Physiol 27:59 –75. Budelmann BU, Bleckmann H. 1988. A lateral line analogue in cephalopods: water waves generate microphonic potentials in the epidermal head lines of Sepia and Lolliguncula. J Comp Physiol A 164:1–5. Burighel P, Cloney RA. 1997. Urochordata: Ascidiacea. In: Harrison FW, Ruppert EE, editors. Microscopic anatomy of invertebrates, vol. 16. Hemichordata, Chaetognatha and the invertebrate chordates. New York: Wiley-Liss. p 221–347. Burighel P, Lane NJ, Zaniolo G, Manni L. 1998. Neurogenic role of the neural gland in the development of the ascidian, Botryllus schlosseri. J Comp Neurol 394:230 –241 Burighel P, Sorrentino M, Zaniolo G, Thorndyke MC, Manni L. 2001 The peripheral nervous system of an ascidian, Botryllus schlosseri, as revealed by cholinesterase activity. Invert Biol 120:185–198. Clarke JDW. 1999. Using fluorescent dyes for fate mapping, lineage analysis, and axon tracing in the chick embryo. In: Sharpe PT, Mason I, editors. Molecular embryology. Methods and protocols. Totowa, NJ: Humana Press. p 319 –328. Day EC. 1919. The physiology of the nervous system of the tunicate. I. The relation of the nerve ganglion to sensory response. J Exp Zool 28:307– 335. Derby CD, Steullet P. 2001. Why do animals have so many receptors? The role of multiple chemosensors in animal perception. Biol Bull 200:211– 215. Dominguez P, Jacobson AG, Jefferies RPS. 2002. Paired gill slits in a fossil with a calcite skeleton. Nature 417:841– 844. Fay RR, Popper AN. 2000. Evolution of hearing in vertebrates: the inner ears and processing. Hear Res 149:1–10. Fedele M. 1920. Nuovo organo di senso nei Salpidae. Monit Ital Zool 31:10 –17. Fedele M. 1923. Sulla organizzazione e le caratteristiche funzionali dell’attività nervosa dei Tunicati. I. Ricerche sul sistema nervoso periferico degli Ascidiacea. Rend Accad Naz Lincei Roma Ser 5 32:98 –102. Fenaux R. 1998. Anatomy and functional morphology of the Appendicularia. In: Bone Q., editor. The biology of pelagic tunicates. Oxford: Oxford University Press. p 25–34. Finger TH. 1997. Evolution of taste and solitary chemoreceptor cell systems. Brain Behav Evol 50:234 –243. Fritzsch B, Beisel KW. 2001. Evolution of the nervous system. Evolution and development of the vertebrate ear. Brain Res Bull 55:711–721. Gallagher BC, Henry JJ, Grainger RM. 1996. Inductive processes leading to inner ear formation during Xenopus development. Dev Biol 175:95– 107. Gautron J 1982. Ultrastructural localization of acetylcholinesterase. Histochemistry 76:469 – 478. SECONDARY SENSORY CELLS IN ASCIDIANS Gillespie PG. 1995. Molecular machinery of auditory and vestibular transduction. Curr Opin Neurobiol 5:449 – 455. Goodbody I. 1974. The physiology of ascidians. Adv Mar Biol 12:1–149. Graham A. 2000. The evolution of the vertebrates — genes and development. Curr Opin Genet Dev 10:624 – 628. Groves AK, Bronner-Fraser M. 2000. Competence, specification and commitment in otic placode induction. Development 127:3489 –3499. Hecht S. 1918a. The physiology of the Ascidia atra Leseuer. I. General physiology. J Exp Zool 25:229 –259. Hecht S. 1918b. The physiology of the Ascidia atra Leseuer. II. Sensory physiology. J Exp Zool 25:261–299. Hellmann B, Fritzsch B. 1996. Neuroanatomical and histochemical evidence for the presence of common lateral line and inner ear efferents to the basilar papilla in a frog, Xenopus laevis. Brain Behav Evol 47:185– 194. Holland LZ, Holland ND. 2001. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate? J Anat 199:85–98. Holland ND, Yu JK. 2002. Epidermal receptor development and sensory pathways in vitally stained amphioxus (Branchiostoma floridae). Acta Zool (Stockh) 83:309 –319. Hudspeth AJ. 1997. Mechanical amplification of stimuli by hair cells. Curr Opin Neurobiol 7:480 – 486. Jefferies RPS. 2001. The origin and early fossil history of the chordate acoustico-lateralis system, with remarks on the reality of the echinoderm-hemichordate clade. In: Ahlberg PE, editor. Major events in vertebrate evolution. London: Systematics Association and Taylor & Francis. p 44 – 66. Katz MJ. 1983. Comparative anatomy of the tunicate tadpole, Ciona intestinalis. Biol Bull 164:1–27. Koppl C. 2001. Efferent axons in the avian auditory nerve. Eur J Neurosci 13:1889 –1901. Kozmik Z, Holland ND, Kalousova A, Paces J, Schubert M, Holland LZ. 1999. Characterization of an amphioxus paired box gene, AmphiPax2/ 5/8: developmental expression patterns in optic support cells, nephridium, thyroid-like structure and pharyngeal gill slits, but not in the midbrain-hindbrain boundary region. Development 126:1295–1304. Lacalli TC. 2001. New perspectives on the evolution of protochordate sensory and locomotory systems, and the origin of brains and heads. Philos Trans R Soc Lond B 356:1565–1572. Lacalli TC, Hou S. 1999. A reexamination of the epithelial sensory cells of amphioxus (Branchiostoma). Acta Zool (Stockh) 80:125–134. Lacalli TC, Gilmour THJ, Kelly SJ. 1999. The oral nerve plexus in amphioxus larvae: function, cell types and phylogenetic significance. Proc R Soc Lond B 266:1461–1479. Lane NJ, Dallai R, Burighel P, Martinucci GB. 1986. Tight and gap junctions in the intestinal tract of tunicates (Urochordata): a freezefracture study. J Cell Sci 84:1–17. Mackie GO. 1995. On the “visceral nervous system” of Ciona. J Mar Biol Assoc UK 75:141–151. Mackie GO, Singla CL. 2003. The capsular organ of Chelyosoma productum 249 (Ascidiacea: Corellidae): a new tunicate hydrodynamic sense organ. Brain Behav Evol 61:45–58. Mackie GO, Wyeth RC. 2000. Conduction and coordination in deganglionated ascidians. Can J Zool 78:1626 –1639. Manley GA, Koppl C. 1998. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol 8:468 – 474. Manni L, Lane NJ, Sorrentino M, Zaniolo G, Burighel P. 1999. Mechanism of neurogenesis during the embryonic development of a tunicate. J Comp Neurol 412:527–541. Manni L, Lane NJ, Burighel P, Zaniolo G. 2001. Are neural crest and placodes exclusive to vertebrates? Evol Dev 3:1–2. Manni L, Lane NJ, Zaniolo G, Burighel P. 2002. Cell reorganization during epithelial fusion and perforation: the case of ascidian branchial fissures. Dev Dyn 224:303–313. Martinucci GB, Dallai R, Burighel P, Casagrande L. 1992. Ciliary specializations in branchial stigmatal cells of protochordates. Tissue Cell 24:229 –241. Millar RH. 1953. Ciona. LMBC Memoirs. Colman JS, editor. Liverpool: University Press. Northcutt RG, Gans C. 1983. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol 58:1–28. Sabbadin A. 1955. Osservazioni sullo sviluppo, l’accrescimento e la riproduzione di Botryllus schlosseri (Pallas), in condizioni di laboratorio. Boll Zool 22:243–263. Seeliger O. 1893–1906. Tunicata. In: Bronn’s Klassen und Ordnungen des Tier-Reichs, Bd 3 (suppl.), Abt. 1, p 1–1280. Shimeld MS, Holland PW. 2000. Vertebrate innovations. Proc Natl Acad Sci USA 97:4449 – 4452. Smotherman MS, Narins PM. 2000. Hair cells, hearing and hopping: a field guide to hair cell physiology in the frog. J Exp Biol 203:2237–2246. Sorrentino M, Manni L, Lane NJ, Burighel P. 2000. Evolution of cerebral vesicles and their sensory organs in an ascidian larva. Acta Zool (Stockh) 81:243–258 Takasaka T, Smith CA. 1971. The structure and innervation of the pigeon’s basilar papilla. J Ultrastruct Res 35:20 – 65. Thurm U, Brinkmann M, Golz R, Lawonn P, Oliver D. 1998. The supramolecular basis of mechanoelectric transduction studied in concentric hair bundles of invertebrates. In: Taddei-Ferretti C, Musio C, editors. From structure to information in sensory system. London: World Scientific. p 228 –236. Wada H. 1998. Evolutionary history of free-swimming and sessile lifestyles in Urochordates as deduced from 18S rDNA molecular phylogeny. Mol Biol Evol 15:1189 –1194. Wada H, Holland PWH, Satoh N. 1996. Origin of patterning in neural tubes. Nature 384:123. Wada H, Saiga H, Satoh N, Holland PWH. 1998. Tripartite organization of the ancestral chordate brain and the antiquity of placodes: insights from ascidian Pax-2/5/8, Hox and Otx genes. Development 125:1113– 1122. Zaniolo G, Lane NJ, Burighel P, Manni L. 2002. Development of the motor nervous system in ascidians. J Comp Neurol 443:124 –135.










![[SENSORY LANGUAGE WRITING TOOL]](http://s1.studyres.com/store/data/014348242_1-6458abd974b03da267bcaa1c7b2177cc-150x150.png)