* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
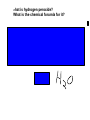
Download What is hydrogen peroxide?
Survey
Document related concepts
Transcript
Unit Three Elements and the Periodic Table HE ATOM T The smallest part of an element that has properties of that element. In 1981, a type of microscope called a scanning tunneling microscope (STM) was developed. STM image of a single zigzag chain of cesium atoms (red) on a galliumarsenside surface (blue) Photo courtesy National Institute of Standards and Technology (NIST) <http://www.howstuffworks.com/framed.ht m?parent=at om.htm&url=http://nist.gov> Copper atoms nanotechweb.org/article s/ne ws/3/9/8/1/niststm1 MODEL A way scientists explain something unknown by relating it to something that can be seen or understood. What are some reasons scientists use models? You have certainly had experience with models in science in your past. Name one of the models you have used in science class. Why is our current understanding of the atom still considered a model or a theory? Model of an atom. Empedocles (49- 432 BCE) argued that all matter was composed of four elements: fire, air, water, and earth. The ratio of these four elements affected the properties of the matter. Atomic Model Timeline Democritus 2500 years ago First idea of an atom "atomos" (invisible) Democritus He suggested that atomos were eternal and could not be destroyed. Democritus theorized that atomos were specific to the material that they made up, meaning that the atomos of stone were unique to stone and different from the atomos of other materials, such as fur. This was a remarkable theory that attempted to explain the whole physical world in terms of a small number of ideas. Alchemy Middle Ages, 1200-1600 Goal was to change cheap elements into gold Philosopher’s Stone The philosophers' stone is a legendary alchemical substance, said to be capable of turning base metals , especially lead , into gold; it was also sometimes believed to be an elixir of life , useful for rejuvenation and possibly for achieving immortality . The Scientific Revolution 1600-1700’s Copernicus-the Sun is the center of the Solar System. Galileo-advanced telescopes & experimental physics NewtonLaws of Motion & Gravity John Dalton early 1800’s “Father of the Atomic Theory” John Dalton School Teacher from England 19th Century (1807) He combined the idea of elements with the Greek theory of atoms. Dalton’s Atomic Theory All matter is made of atoms Atoms can’t be created or destroyed All atoms of one element are the same Atoms of different elements can combine to form new substances John Dalton pictured the atom as a hard sphere with the same makeup throughout the entire atom. William Crookes English Scientist 1870's Tested Dalton's theory of the atom. Experimented with an airless glass tube with two metal pieces hooked to a battery. Cathode ray tube + CRT Same type of mechanism found in tv's and computer monitors Saw a "beam" of something from the negatively charged cathode to the positively charged anode. The beam looked like a grennish-yellowish light, but Crookes said it was a beam of particles. He couldn't prove it!!! (bummmer!) J. J. Thomson 1897 English physicist Worked on Crooke's experiments. Use a magnet next to the glass tube. Proved that the beam was not light...Light can not be bent with a magnet. The beam bent towards the magnet.....Opposite charges attract. Knew that the particles were negatively charged. HOW? Named the negatively charged particles ELECTRONS. "Plum Pudding Model of the Atom" J J Thomson's model of the atom. Your assignment: write in the symbols for each of the elements, and learn them. Quiz on the elements will be next week. Element Name Symbol Aluminum _____ Antimony _____ Argon _____ Arsenic _____ Barium _____ Beryllium _____ Bismuth _____ Boron _____ Bromine _____ Calcium _____ Carbon _____ Chlorine _____ Chromium _____ Cobalt _____ Copper _____ Fluorine _____ Gallium _____ Germanium _____ Gold _____ Helium _____ Hydrogen _____ Iodine _____ Iron _____ Krypton _____ Lead _____ Element Name Symbol Lithium _____ Magnesium _____ Manganese _____ Mercury _____ Neon _____ Nickel _____ Nitrogen _____ Oxygen _____ Phosphorus _____ Platinum _____ Plutonium _____ Potassium _____ Radon _____ Selenium _____ Scandium _____ Silicon _____ Silver _____ Sodium _____ Sulfur _____ Tin _____ Titanium _____ Tungsten _____ Uranium _____ Vanadium _____ Zinc _____ Element Name Symbol Aluminum Al Antimony Sb Argon Ar Arsenic As Barium Ba Beryllium Be Bismuth Bi Boron B Bromine Br Calcium Ca Carbon C Chlorine Cl Chromium Cr Cobalt Co Copper Cu Fluorine F Gallium Ga Germanium Ge Gold Au Helium He Hydrogen H Iodine I Iron Fe Krypton Kr Lead Pb Element Name Symbol Lithium Li Magnesium Mg Manganese Mn Mercury Hg Neon Ne Nickel Ni Nitrogen N Oxygen O Phosphorus P Platinum Pt Plutonium Pu Potassium K Radon Rn Selenium Se Scandium Sc Silicon Si Silver Ag Sodium Na Sulfur S Tin Sn Titanium Ti Tungsten W Uranium U Vanadium V Zinc Zn Ernest Rutherford 1906 considered the father of nuclear physics Won the 1908 Nobel Prize in Chemistry Wanted to see if Thomson's model of the atom was correct...... Set up an experiment that fired fast moving positively charged bits of matter (alpha particles) at a thin piece of a metal such as gold. Alpha partices come from unstable atoms. They are positively charged, and so they are repelled by particles of matter which have a positive charge (opposites attract, like repell). Rutherford hypothesized that the gold film did not contain enough matter to stop the speeding alpha particle or change its path........... The unexpected results of the experiment caused Rutherford to realize that Thomson's model was incorrect, and he proposed his own model. Rutherford's model included a nucleus in the atom. The positively charged proton is located in the very small space at the center of an atom. Most of the atom is empty space occupied by nearly massless electrons. Electrically neutral particles, neutrons are also located in the nucleus. The number of electrons equal the number of protons in an atom. Neils Bohr 1913 Electrons are in energy levels Erwin Schrodinger 1926 Electron Cloud Model electron orbitals shapes have James Chadwick 1932 Discovered the neutron Nuclear Power First atomic bomb-1945 Atomic Energy The secrets of the atom’s nucleus is unveiled The science of atomic radiation, atomic change and nuclear fission was developed from 1895 to 1945, much of it in the last six of those years. Over 1939-45, most development focused on the atomic bomb. was From 1945 attention was given to harnessing this energy in a controlled fashion for naval propulsion and for making electricity. Since 1956 the prime focus has been on the technological evolution of reliable nuclear power plants. Uranium was discovered in 1789 by Martin Klaproth, a German chemist, and named after the planet Uranus. Hints for Learning the Element Symbols Au - Aey U ! Give me my gold ! -or- Autumn leaves turn to gold. Na - Na, I don’t want sodium on my food. (think of salt, NaCl) Ag - Aged people have hair of silver. Fe - Females need iron. Hg - The planet Mercury is huge. Pb - Plumbers use lead pipes. Cu - C U (see you) later, copper ! Sn – Cut tin with tin-snips. Pu – P U ! Plutonium stinks. Sb – Antimony is so bad for you. The symbol is the first two letters of the element name. Al Aluminum Ar Argon Ba Barium Be Beryllium Bi Bismuth Br Bromine Ca Calcium Co Cobolt Ga Gallium Ge Germanium He Helium Kr Krypton Li Lithium Ne Neon Ni Nickel Sc Selenium Sc Scandium Si Silicon Ti Titanium The element symbol is only the first letter of the element name. B Boron C Carbon F Fluorine H Hydrogen I Iodine N Nitrogen O Oxygen P Phosphorous S Sulfur U Uranium V Vanadium The symbol is from a foreign The symbol is the first letter of the word for that element, usually element name plus one other letter in Greek or Latin. that name. Sb Antimony Au Gold Cl Chlorine Ag Silver Cr Chromium K Potassium As Arsenic Hg Mercury Mg Magnesium Na Sodium Mn Manganese W Tungsten Pt Platinum Sn Tin Pu Plutonium Pb Lead Rn Radon Fe Iron Zn Zinc Cu Copper Gold Palladium Iron Silver Mercury Copper Zinc Krypton Aluminum Lithium Sodium Neon Helium Magnesium Nikel Fluorine Tin Coblt Lead Bariium Bromine Sodium Sulfur Argon Chlorine Radium Silicon Calcium Radon Fluorine Beryllium Chlorine Plutonium Iodine Boron Li He Pb Ag Ca Li S Ne Os Kr Cl Ba Pu P F K Co N Sr Sn Fe Si Cu C Br Al Hg Be O H Rn Ra He Mg Au Element symbol test tomorrow! Use your flash cards and be ready for it!! Welcome Mr. Koy! What is hydrogen peroxide? What is the chemical forumla for it? Hydrogen peroxide is an oxidizer commonly used as a bleach. It is a clear liquid , slightly more viscous than water, that appears colorless in dilute solution. It is used as a disinfectant , antiseptic , oxidizer, and in rocketry as a propellant. The oxidizing capacity of hydrogen peroxide is so strong that it is considered a highly reactive oxygen species . H2O2 Lab: Observing the element OXYGEN Hour ___________ Date __________ Objective: To collect and observe a sample of oxygen. Materials: Safety glasses, 2 standard test tubes, test tube rack, 3 wood splints, manganese dioxide (MnO2), distilled water (H2O), hydrogen peroxide (H2O2), matches, graduated cylinder (10 mL), test tube clamp Procedure: Use CLEAN, DRY test tubes. With a pencil, mark one tube “w” for water. Mark the other tube “h” for hydrogen peroxide. Put 7 mL distilled water in the tube marked “w”. Put 7 mL hydrogen peroxide in the test tube marked “h”. 3. Complete the table below. Substance Water Hydrogen Peroxide State of matter Color Observable differences? 4. Use a wooden splint to add the manganese dioxide to each of the two test tubes. Put in 1 splint-full. Record your observations below. Substance Observations when mixed with MnO2 Water Hydrogen Peroxide 5. Use a new wood splint. Light the splint with a match. Let the splint burn 3 seconds, then blow out the flame. A small area on the splint will continue to glow orange. Move the glowing splint down into the tube marked “w,” but DO NOT let it touch the liquid. This is called a splint test. Observe and record below. 6. Add one more splint-full of manganese dioxide to the “h” tube. Put your thumb over the test tube and shake gently. Repeat the splint test procedure on this tube. Observe any differences in the glowing splint carefully. Record below. Substance Water Hydrogen Peroxide Formula Splint test observation s Model/Drawi ng QUESTIONS TO ANSWER Look at the formulae for water and hydrogen peroxide. What elements are in each of these compounds? __________________________ and _______________________________ Which substance, water or hydrogen peroxide, has more oxygen atoms? ___________________ Oxygen is the gas that bubbled out of the tube with hydrogen peroxide. Write two properties of oxygen. ____________________________________ ______________________________________ The air we breathe contains about 19% oxygen, 79% nitrogen, and 2% carbon dioxide. Is air a MIXTURE or an ELEMENT? __________________________________ Oxygen is one of those elements sometimes confused when students write chemical symbols. Using a lower case letter for the second letter of an element’s abbreviation or symbol is important. Write the element or elements shown by each of the symbols below. Co _______________________________ C O _____________________ ________________________ P O _____________________ ________________________ Pu _______________________________ P U _____________________ _______________________ S I ______________________ _______________________ Si _______________________________ Ni _______________________________ N I _____________________ _______________________ C U ____________________ _______________________ Cu ______________________________ Please use a complete sentence to explain one thing you learned by doing this lab today. _____________________________________________________________________________________________ __________________ _____________________________________________ ________________________________________________