* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sodium
Geomorphology wikipedia , lookup
Large igneous province wikipedia , lookup
History of Earth wikipedia , lookup
Algoman orogeny wikipedia , lookup
History of geology wikipedia , lookup
Tectonic–climatic interaction wikipedia , lookup
Provenance (geology) wikipedia , lookup
Age of the Earth wikipedia , lookup
Composition of Mars wikipedia , lookup
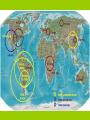
Mineralogy and Geochemistry Lecture: Sándor Szakáll Practice: Ferenc Móricz Aim of the course • Distribution and abundance of chemical elements in the spheres of the Earth, especially: appearance in the minerals (solid, crystalline compounds, elements). • Chemical elements in magmatic, sedimentary and metamorphic processes, positions in the structure of the minerals. Predominant, substituting and trace elements in minerals. • Positive anomalies, enrichments of chemical elements in magmatic, sedimentary and metamorphic processes. • Appearances of the elements in hydrosphere and biosphere Programme 1) Hydrogen and alkaline metals 2) Alkaline earth metals 3) Boron, aluminium, carbon, silicon 4) Rare earth elements, titanium, zirconium 5) Uranium, thorium, vanadium, niobium, tantalum 6) Chromium, molybdenium, tungsten 7) Manganese, iron, cobalt, nickel 8) Copper, gold, silver, platina group elements 9) Zinc, cadmium, mercury, gallium, indium, thallium 10) Tin, lead, arsenic, antimony, bismuth 11) Nitrogen, phosphorus, oxygen 12) Sulphur, selenium, tellurium, haloids, noble gases Hydrogen (H) Universe: 7.5 x 105 ppm (by weight) Sun: 7.5 x 105 ppm (by weight) Carbonaceous meteorite: 24000 ppm Earth's Crust: 1500 ppm Seawater: 107800 ppm Carbonaceous meteorites: their compositions are considered to be close to that of the solar nebula from which the Solar system condensed. Hydrogen in the lithosphere 1) Enrichment in the late period of magmatic processes: especially in pneumatolithic, and hydrothermal system (in the fluids and aqueous solutions). In volcanic vapor (fumarolae) as water, or in haloids as HCl, HF or H2S. 2) In the structure of minerals: hydroxil group as anions (hydroxiles/oxi-hydroxiles) or additional anions (e.g. amphiboles, micas, alunite-jarosite minerals); in loosely bonded water molecules of the crystal structure (some sulphates, phosphates, arsenates, zeolites, clay minerals, especially smectites etc.), as independent H+ ions in rare salt minerals. 3) Water molecules in porus of the rocks. In rock-forming quantities as ice. The ice really one of the most important mineral of hydrogen. Hydrogen in the hydrosphere, atmosphere, and biosphere It concentrates largest quantities as water. Lesser amounts in volcanic vapors (HCl, H2S, NH3 etc.). Continous cycle of water between litosphere, hydrosphere, atmosphere. Geochemically importance of water in the chemical alteration, diagenesis, and soil formation. H+ ions occurs in the aqueous solutions. As H2 gas molecules found in the atmosphere. Hydrogen (and carbon) main constituent of organic compounds. Some of them have crystalline state (they are the organic minerals). Essencially component of hydrocarbons. The water always contains some compounds in solution state. The ratio of these components depends of the chemical compounds of the host-rocks This world map shows how the salinity of the oceans changes slightly from around 32ppt (3.2%) to 40ppt (4.0%). Low salinity is found in cold seas, particularly during the summer season when ice melts. High salinity is found in the ocean 'deserts' in a band coinciding with the continental deserts. Due to cool dry air descending and warming up, these desert zones have very little rainfall, and high evaporation. The Red Sea located in the desert region but almost completely closed, shows the highest salinity of all (40ppt) but the Lithium (Li) Universe: 0.006 ppm (by weight) Sun: 0.00006 ppm (by weight) Carbonaceous meteorite: 1.7 ppm Earth's Crust: 20 ppm Seawater: 0.18 ppm Lithium in magmatic processes It is enriched in ferromagnesium minerals (e.g. amphiboles, pyroxenes, see Li-type inosilicates), but it does not substitutes in feldspars structure. It mainly replaces Mg or Fe2+ (ionic radius of Li+ 0.68, Mg2+ 0.66, Fe2+ 0.74). It concentrated in later stages of magmatic differentiation (pneumatolithic, pegmatitic), and forms separate phases, as spodumene, petalite, lepidolite, zinnwaldite, elbaite. About micas (e.g. biotite, muscovite) it does not replaces alkali metals, Na or K (than feldspars), but it substitutes Al or Mg, too. The largest postmagmatic accumulations have pegmatitic or pneumatholitic origin with Li-bearing silicates. Lithium in weathering and sediments Lithium is readily absorbed by clay minerals during weathering. In this processes it moves similarly than Mg. It can accumulates in evaporites or claystones. The Li/Mg ratio is 0.0034 in magmatic rocks, while 0.00008 in seewater (the reason of difference is the absorption facility in clays). It appears dissociated Li+Cl- forms in thermal waters, and mineral waters. There are three types of brine deposit (continental, geothermal and oil-field) with the most common being continental saline desert basins. They are located near tertiary or recent volcanoes and are made up of sand, minerals with brine and saline water with high concentrations of dissolved salts. In clay deposits, lithium is found mainly in the mineral smectite (hectorite variety). Lithium in water The total lithium content of seawater is very large and is estimated as 230 billion tonnes, where the element exists at a relatively constant concentration of 0.14 to 0.25 parts per million (ppm). However, lithium is present in seawater, commercially viable methods of extraction have yet to be developed. One potential source of lithium is the leachates of geothermal wells, which are carried to the surface. Recovery of lithium has been demonstrated in the field. The lithium is separated by simple filtration. Sodium (Na) Universe: 20 ppm (by weight) Sun: 40 ppm (by weight) Carbonaceous meteorite: 5600 ppm Earth's Crust: 23000 ppm Seawater: 11500 ppm Potassium (K) Universe: 3 ppm (by weight) Sun: 4 ppm (by weight) Carbonaceous meteorite: 710 ppm Earth's Crust: 15000 ppm Seawater: 450 ppm Sodium and potassium in magmatic rocks Sodium is a major element in the Earth, especially in crustal rocks. The bulk Earth Na content is variously estimated to be about 1.6-1.8 mg/g. Sodium is an essential constituent of many rock-forming minerals (there are almost silicates), where it is typically in either 6- or 8-fold coordination. Common minerals in which Na is an essential constituent include plagioclase (felsic part: albite), paragonite, Naamphiboles, Na-pyroxenes (aegirine), nepheline, sodalite, zeolites (analcime, natrolite), Na-tourmalines. Under high pressure, Na can substitute in pyroxene as the jadeite component, NaAlSi2O6. Some alkali magmatic rocks contain other exotic Na minerals, e.g. NaF, villiaumite, pectolite. Sodium and potassium in magmatic rocks Common minerals in which potassium is an essential constituent include alkali feldspars (orthoclase, microcline, sanidine), leucite, biotite series, muscovite, phlogopite. Sodium and potassium are volatile lithophile elements and are monovalents. Both elements concentrated in the upper continental crust of the Earth. They concentrate highly amounts in alkali magmatic rocks (one of Na and K series in which nepheline, leucite and the alkali amphiboles/pyroxenes are the index minerals). The Na/K ratio decrease from basic to acid magmatites. Potassium, like sodium, is enriched acidic magmas. Basic and ultrabasic rocks, which are rich in iron and magnesium, contain little potassium, and sodium. Sodium and potassium in weathering During weathering of rocks, Na and K liberated by mineral breakdown readily dissolves in the weathering solutions and enters to the hydrosphere. Like all of the alkali elements, Na and K ions are highly soluble in aqueous solutions. In contrast to sodium, potassium migrates only slightly on the earth’s surface. Weathering of rocks leads to partial transfer of potassium to water, but this is rapidly absorbed by organisms and clay minerals; therefore, river waters are poor in potassium and much less potassium than sodium reaches the oceans. In the ocean, potassium is absorbed by organisms and bottom silts (e.g. a main component of glauconite). For this reason ocean waters contain only 0.038 percent potassium (25 times less than sodium). Sodium and potassium in weathering The difference in abundance of K and Na in sediments or surface waters is the difference of K and Na appearance in the structure of clay minerals (it is much more Kbearing clay minerals than Na-bearing ones). See illite, glauconite. Plus, the difference of weathering features sodiumfeldspars and plagioclases (see table). Finally, better absorption features of potassium than sodium (see soil minerals, or manganese oxides/hydroxides). Sodium and potassium in evaporites The largest sources of Na and K are in the evaporites (chlorides, sulphates, rarely borates, nitrates). They form by evaporation from salt-containing waters. Seewater and freshwater differ about cation/anion compositions, so the non-marine evaporitic mineral asseblages are far more diverse than marine assemlages. The non-marine evaporites consist of mainly Na-salts (chlorides, halite, carbonates, thermonatrite, natron, trona, and sulphates, mirabilite, thenardite), in contrary to marine evaporites, in which many K-salts can accumulate. There are 5 typical Naassociations: 1) Na-CO3-Cl; (2) Na-CO3-SO4CI; (3) NaSO4CI; (4) Na-Mg-SO4Cl; (5) Ca-Mg-Na-Cl. Sodium and potassium in biosphere Na and K not only lithophil, but definitely biophil elements, too. Na is characteristic microcomponent of plants (it occurs higher amounts in marine plants). It is the main cation in body fluids in the animals and the man. Sodium is an essential mineral that regulates blood volume, blood pressure, osmotic equilibrium and pH. Because of ion equilibrium very important the fixation of Na/K ratio in the organics. The play of K is similar than Na in organics, but rather in plants (e.g. synthesis of chlorophyl). It plays an important role in the physical fluid system of humans and it assists nerve functions. As the K+, concentrate inside cells, and 95% of the body's potassium is so located. Rubidium (Rb) Universe: 0.01 ppm (by weight) Sun: 0.03 ppm (by weight) Carbonaceous meteorite: 3.3 ppm Earth's Crust: 60 ppm Seawater: 0.12 ppm Cesium (Cs) Universe: 0.0008 ppm (by weight) Sun: 0.008 ppm (by weight) Carbonaceous meteorite: 0.14 ppm Earth's Crust: 3 ppm Seawater: 3 x 104 ppm Rubidium in magmatic processes Rubidium is strongly incompatible with almost all mantle minerals (except phlogopite), and is therefore strongly enriched in the continental crust relative to the mantle (overall crustal abundance ~32 ppm). It is suggest that rubidium is strongly depleted in the lower crust relative to the upper crust ( 5.3 ppm vs 112 ppm). It appears mainly in the late-stage stadiums, pneumatolithic and pegmatitic. It substitutes K in silicate structures (alkali feldspars, micas). In the late-stage magmatic products, such as pegmatites, tend to have extremely high concentrations of Rb (> 1000 ppm). The Rb has only one mineral, as rubicline (it occurs close association with microcline). Cesium in magmatic processes Because of low abundance in Earth crust, Cs does not form any common rock-forming minerals. Rather, Cs substitutes for K in rock-forming minerals (e.g. in alkali feldspars, biotite and muscovite). The Cs contents of typical granites are of the order of 1300-6000 ng/g. Hence, Cs is concentrated in the upper continental crust of the Earth. It mainly accumulates in pegmatites, where it has some independent minerals, among them the most important is the pollucite (a tectosilicate). Rubidium and cesium in weathering and sedimentary rocks The behavior of rubidium in sedimentary processes is controlled by the adsorption of Rb onto clay minerals (illite and montmorillonite), which adsorb Rb+ more strongly than K+. In contrary to Rb not incorporated in significant amounts into carbonate minerals. So, they behave than K in sedimentary processes, absorption in clays, or replace K in evaporite minerals. During weathering of rocks, Cs readily dissolves in the weathering solutions and enters to the hydrosphere. Like all alkali elements, Cs ions are highly soluble in aqueous solutions.