* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ideas to Implementation by Ian Wilkinson
Time in physics wikipedia , lookup
Electromagnet wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Condensed matter physics wikipedia , lookup
Lorentz force wikipedia , lookup
Electric charge wikipedia , lookup
Electrostatics wikipedia , lookup
Electromagnetism wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Superconductivity wikipedia , lookup
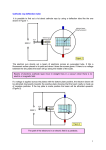
HSC Physics Module 9.4 Summary 1. Increased understanding of cathode rays led to the development of television Identify that moving charged particles in a magnetic field experience a force Recall that a current carrying conductor experiences a force in a magnetic field, and that current is the movement of charges The moving charges in the conductor in the magnetic field cause the conductor to experience a force Generally, any charge or collection of charges moving relative to a magnetic field will experience a force Identify that charged plates produce an electric field Recall that an electric field is a region where charged objects will experience a force, and that a point charge or collection of charges will produce an electric field When two charged plates are brought together, they will exert a force on each other, thus an electric field exists between the plates See below for diagrams of the electric field between two oppositely-charged plates. Describe quantitatively the force acting on a charge moving through a magnetic field As mentioned above, a charge moving through a magnetic field will experience a force The magnitude of the force a moving charge experiences in a magnetic field is given by: where: o F = Force [N] o q = Charge [C] Note: 1 coulomb = 6.24x1018 elementary (electron) charges o v = velocity [ms-1] o B = magnetic field strength [Tesla] o Θ = angle between the velocity and the direction of the magnetic field lines The term sinΘ is included because only the component of the charge’s velocity perpendicular to the magnetic field is subject to a force The direction of the force can either be given by either the right-hand-palm rule or general right-hand rule o o For the right-hand-palm rule, point the thumb in the component of velocity of a POSITIVE charge perpendicular to the magnetic field (opposite direction for a negative charge), and the fingers in the direction of the magnetic field. The palm will point in the direction of the force. For the right-hand rule, point the fingers in the component of velocity of a positive charge perpendicular to the magnetic field, and curl fingers in the direction of the magnetic field. The thumb will point in the direction of the force. Discuss qualitatively the electric field strength due to a point charge, positive and negative charges and oppositely charged parallel plates The strength and direction of electric fields can be represented by flux lines. The strength of an electric field is the relative magnitude of force a charged particle would experience in the field The direction of an electric field is defined as the direction a positive charge would experience a force in the field o Thus a negative charge would move in the opposite direction to direction of the flux lines if it were placed in an electric field A number of rules apply to the interpretation of electric field diagrams using flux lines: o Flux lines begin on positive charges and end on negative charges o Flux lines never cross o Flux lines enter and exit at right angles only o Flux lines that are close together represent strong fields o Flux lines that are well-separated represent weak fields o A negative charge placed in the field will experience a force in the direction opposite to the arrow of the flux lines FIELDS OF POINT CHARGES The field strength of a point charge obeys the inverse square law, thus the field strength decreases proportionally to the inverse square of the distance to the charge o Thus we can consider the electric field around a point charge to be radial Point charges with a stronger charge produce stronger electric fields, which are represented by closer field lines in electric field diagrams When multiple charges are placed closed together, the resultant field is the superposition of the fields of each charge The diagram below shows the electric fields of point charges of equal/opposite charge The diagram below shows the superposition of electric fields of charges of different magnitudes of charge FIELDS OF OPPOSITELY-CHARGED PARALLEL PLATES Oppositely-charged parallel plates, also known as parallel-plate capacitors, produce uniform electric fields If the area of the parallel plates is significantly greater than the distance between the plates, the resulting electric field will be uniform, except at the edges where it slightly bulges When drawing the electric field between two parallel plates, remember the following: o The flux lines must be evenly spaced o The flux lines should bulge at the edge of the plates o The flux lines should go from the positively-charged plate to the negatively-charged plate Below is a diagram of the field between two oppositely-charged parallel plates Describe quantitatively the electric field due to oppositely charged parallel plates Recall that the magnitude of an electric field is equal to the force per unit charge at a point in the field, given by OR Where o E = electric field strength [NC-1] o q = charge [C] o F = force on the charged object [N] Recall that voltage is the change in potential energy or work done on charge per unit charge, given by OR But work also equals force multiplied by distance. Therefore… Equating the two expressions for work, we get Dividing be q and rearranging we get Where o E = electric field strength in between the plates [Vm-1] o V = potential difference between the plates [V] o d = distance between the plates [m] Thus the electric field between two oppositely-charged parallel plates can be calculated by considering the potential difference and distance between the two plates When using the above formula, quote the units as Vm-1 rather than NC-1. Whilst both units are equal, quote the units for field strength according to the equation used. Thus we can see from the above formula that the electric field strength is… o Proportional to the potential difference between the plates o o o Inversely proportional to the distance between the plates Equal at all points between the plates Perpendicular to the plates everywhere in the region between the plates Solve problems and analyse information using: ALWAYS specify the direction of any vectors, including force, electric field, and magnetic field Ensure all calculations include dimensions and are dimensionally correct IMPORTANT NOTES Use your right-hand when working out the direction Crosses mean that the field is INTO the page, points mean OUT of page When calculating the force an electron experiences in either a magnetic field, reverse the direction of velocity when working out the direction of force When calculating the force an electron experiences in an electric field, ensure the force is in the direction opposite to a magnetic field Explain why the apparent inconsistent behaviour of cathode rays caused debate as to whether they were charged particles or electromagnetic waves Cathode rays were observed during the 19th century in vacuum tubes with two electrodes inside and a voltage applied. Their presence was detected by the glowing glass opposite of the cathode (negative electrode). Scientists determined that the glow was due to a ray emitted from the cathode (hence their name cathode rays), but their properties were inconsistent with both particle and wave motions. The conflicting observations led physicists to become divided on whether cathode rays were particles or electromagnetic waves o For example, Crookes demonstrated that cathode rays were deflected by magnetic fields (which supports the particle model), but Hertz showed that cathode rays weren’t deflected by electric fields (which supports the wave model) o Hertz’s experiment was later shown to be flawed however, as Thomson demonstrated the deflection of cathode rays due to an electric field by using a more complete vacuum than Hertz. At higher gas pressures, the cathode rays ionised the gas, which were attracted to the oppositely charged plates, and neutralised the charge on the plates, thus the rays weren’t deflected in Hertz’s experiment. The following observations supported the wave model: o Cathode rays travelled in straight lines o If an opaque object (such as a Maltese cross) was placed in their path, a shadow of that object appeared o They could pass through thin metal foils without damaging them The following observations supported the particle model: o The rays left the cathode at right angles to the surface o They were deflected by magnetic fields o Small paddlewheels turned when placed in the path of the rays, showing they had momentum and thus mass o They travelled considerably slower than light. The apparent inconsistencies of the behaviour of cathode rays were due to the inadequacies of experimental design and the current state of knowledge about the nature of atoms. o The atom was later shown to be predominately empty space, so small electrons could pass through metal foils without causing damage Thomson showed that the rays were deflected towards the positively charged plate, thus demonstrating that they were negatively charged particles Further experiments showed that cathode rays were a stream of electrons The resolution of the inconsistencies of the behaviour of cathode rays is an example of the scientific method, i.e. observations from experiments are interpreted and a hypothesis developed to explain what is thought to be happening. Opposing models are then resolved through improved experimentation, allowing us to gain a greater understanding of the nature of cathode rays. Explain that cathode ray tubes allowed the manipulation of a stream of charged particles A cathode ray tube is a highly-evacuated, sealed glass tube containing two electrodes. o The negatively-charged electrode is called the cathode, whilst the positively-charged electrode is called the anode o Remember that cations (positive ions) are attracted to the cathode, and anions (negative ions) are attracted to the anode Applying a high voltage across the tube causes cathode rays to be produced, which are streams of negatively-charged particles (electrons) to flow from the cathode to the anode, with little obstruction from collisions with remaining gas particles As cathode rays are negatively-charged, they can be deflected by applying an external electric or magnetic field In addition, placing solid or otherwise objects in the path modifies the path of the beams For example… o Applying an external electric field deflects cathode rays to the positive plate, demonstrating that they are negatively charged (parabolic deflection) o Applying an external magnetic field deflects the cathode rays perpendicularly to the magnetic field (circular deflection) o Placing solid objects in the cathode ray tube inhibits the movements of cathode rays A Maltese cross produces a shadow A paddlewheel rotates when placed in between the anode and cathode Outline Thomson’s experiment to measure the charge/mass ratio of an electron Many of Thomson’s experiments centred on his study of cathode rays, such as his demonstration of the deflection of cathode rays due to an electric field Thomson was also able to determine the charge to mass ratio of an electron through the analysis of cathode rays His experimental set-up is shown below: o o o The glass tube is sealed and at reduced pressure The cathode rays are emitted from the cathode (negatively-charged electrode) The anodes consist of charged plates with thin slits => they act as anode collimators, as the cathode ray accelerates towards the plates, passes through the slit, and enters the main tube as fine and well-defined beam o The charged plates produce an electric field (in exams, mark which one is positive, which is negative, and the direction of the field) o The coils act as electromagnets, and produce a magnetic field o The fluorescent screen allows the cathode rays to be detected His experiment consisted of two steps: o He first varied the magnetic and electric fields until their opposing forces cancelled, leaving the cathode ray undeflected. By equating the magnetic and electric force equations, Thomson was able to determine the velocity of the cathode-ray particles. o He then applied the same strength magnetic field (alone), and determined the radius of the circle path travelled by the charged particles in the magnetic field As v had already been calculated, and r and B can be measured (r by careful observation), Thomson was able to calculate the charge-to-mass ratio of the particles in the cathode ray His experiment demonstrated that cathode rays were particles, because if the cathode ray has a charge-to-mass ratio, the cathode-ray particles must have a measurable mass He calculated that all cathode-ray particles (electrons) had a charge-to-mass ratio of 1.76x1011Ckg-1, regardless of the cathode material, gases, or other conditions o This indicated that the cathode-ray particles (electrons) were common to all materials, which was one piece of evidence indicating that atoms were made of subatomic particles The calculated charge-to-mass ratio was over a thousand times higher than that of a hydrogen ion (H+), suggesting that the particles were either very light or very highly charged. o Such results contributed to the development of Thomson’s plum-pudding model of the atom Outline the role of: electrodes in the electron gun the deflection plates or coils fluorescent screen in the cathode ray tube of conventional TV displays and oscilloscopes Below is a diagram of the cathode ray tube used in oscilloscopes, which is similar to that used in TV displays with a few differences (see below) The three primary components of the cathode ray tube used are the electron gun, the deflection plates or coils, and the fluorescent screen. ELECTRON GUN The electron gun consists of the cathode, anode collimator, and heater The cathode emits the cathode rays, which are accelerated towards to the multiple anodes, and then travel into the deflection part of the tube as a fine, well-defined beam An electrode grid in between the cathode and electrode is used to control the number of electrons reaching the anode, as the grid can be made more positive or negative. This controls the brightness of the display The heater heats up the cathode, which releases many free electrons that can be easily accelerated towards the cathode => this is called thermionic emission DEFLECTION PLATES/COILS The deflection plates or coils produce a unidirectional electric or magnetic field respectively to deflect electrons vertically or horizontally to produce a useful display on the screen o Oscilloscopes use plates to produce an electric field, whilst televisions use coils to produce a magnetic field There are two sets of parallel plates/coils, each set perpendicularly to each other, so that they can deflect the beam both in the vertical and horizontal direction on the screen according to the voltage applied to the plates/coils Thus the deflection plates/coils allow the cathode ray to be deflected to any position on the screen FLUORESCENT SCREEN The fluorescent screen is coated with layers of fluorescent material, which emits light when high energy electrons strike it This allows the position of the beam to be seen where it strikes the screen, and allows a useful image to be formed. Application to the oscilloscope The CRO (Cathode-Ray Oscilloscope) is a diagnostic tool that allows voltage to be plotted against time The horizontally-deflecting plates (X-plates) supply a time-based voltage, so the beam sweeps horizontally across the screen in time intervals that can be controlled as desired The vertically-deflecting plates (Y-plates) are connected to the input voltage (which is amplified as necessary), so their deflection allows the voltage of the input to be measured against time Application to TV displays A colour TV display contains three electron guns, each corresponding to the different colours of red, blue and green The image sent by the signal to the TV is reconstituted on the screen by an additive process involving three coloured phosphors corresponding to red, blue and green. A shadow mask is used on the screen to ensure the beam from each colour gun only hits the corresponding spot on in each pixel, thus forming the correct image The TV display use deflection coils (which produce magnetic fields) to deflect the beam The horizontally- and vertically-deflecting coils are connected to a time-based voltage that scans each line of pixels on the display 50 times a second The phosphors glow for a short time, so no flickering is observable to the retina The colour of each pixel is controlled by the intensity of the beam striking its corresponding phosphor, thus an image is formed Perform an investigation and gather first-hand information to observe the occurrence of different striation patterns for different pressures in discharge tubes METHOD An induction coil was connected to a power supply to supply the high voltages required. Discharge tubes of different pressures were connected to the induction coil, one at a time, and the striation patterns that formed were observed and recorded SAFETY: Take care when handling the discharge tubes, because the low pressures can cause them to easily IMPLODE (not explode) The sparks produced emit X-rays, which are potentially dangerous for prolonged exposure. Stand at least 2m away from the coil whilst it is turned on (consider the inverse square law), and only turn the coil on for short periods of time (no more than 5 seconds) for observation The sparks also produce ozone, which can aggravate respiratory problems => conduct the experiment in a well-ventilated area, and again only turn the induction coil on for short periods of time RESULTS Pressure (mmHg) Striation pattern observed 40 Flashes of purple at anode and cathode Black in the middle 10 Bright purple at anode and cathode Pink stream of light in the body 6 Purple light at anode and cathode One dark gap near cathode Pink-purple body 3 Pink anode Purple flashes at cathode Pink-orange body with striations 0.14 Pink-purple glow at anode and cathode Purple body Less striations Glass fluorescing 0.03 Tube is dark except for the purple fluorescence behind the anode on the glass The glass fluoresced when there was low pressure because there were almost no gaseous molecules for the cathode ray to collide with, so it continued moving up to the glass where it was able to fluoresce it ACCURACY/RELIABILITY/VALIDITY The dot point only asks for observations, so accuracy, reliability and validity are not particularly relevant beyond usual comments Perform an investigation to demonstrate and identify properties of cathode rays using discharge tubes: containing a maltese cross containing electric plates with a fluorescent display screen containing a glass wheel analyse the information gathered to determine the sign of the charge on the cathode rays METHOD An induction coil was connected to a power supply to supply the high voltages required. Four discharge tubes, one containing a Maltese cross, one containing electric plates, one containing a fluorescent display screen, and one containing a glass wheel were connected to the induction coil, one at a time. For the one containing the fluorescent screen, bar magnets were placed around the cathode ray tube, and any observations were noted SAFETY: See practical above RESULTS Discharge tube Observation Maltese cross A shadow of the Maltese cross was projected at the glass near the anode Electric plates The cathode ray was deflected towards the positive plate, detected by the position of the fluorescence near the anode Fluorescent screen and bar magnets The screen fluoresced when the power supply was turned on, leaving a clear trace of itself. The ray was deflected by the presence of bar magnets, as detected on the fluorescent screen Glass wheel The wheel began to spin when the power supply was turned on The shadow of the Maltese cross indicated that cathode rays travel in straight lines The deflection by the magnetic field and the rotation of the glass wheel indicated that cathode rays have momentum, and thus have mass The deflection of the cathode rays towards the positively-charge plate indicated that cathode rays are negatively-charged ACCURACY/RELIABILITY/VALIDITY The cathode ray tubes had very low pressure, thus the deflections observed were accurate and valid The induction coil was turned on and off several times for each tube, and the results matched expected results, so the results were reliable Validity could have been improved by using different discharge tubes, and showing the same effects 2. The reconceptualisation of the model of light led to an understanding of the photoelectric effect and black body radiation Outline qualitatively Hertz’s experiments in measuring the speed of radio waves and how they relate to light waves Maxwell’s developed his theory of electromagnetic waves, in which he proposed that visible light propagated by oscillating electric and magnetic fields without a medium. His equations and calculations implied the following predictions: o Electromagnetic waves could exist with many different frequencies o All electromagnetic waves propagate through space at the speed of light (3.11x108ms-1) Hertz conducted a series of experiments to test and verify these predictions. He produced radio waves by connecting an induction coil to a spark gap and applied a high voltage, which caused current to oscillate across the gap and the rods, thus producing electromagnetic waves. He observed that when a small length of wire, bent into a loop with a small gap, was placed near the sparking induction coil, a spark would synchronously jump across that coil too. Since the loop was placed several metres away from sparking induction coil, he concluded that radio waves caused the sparking in the second coil, thus he was able to use this as a detector of radio waves Hertz determined the frequency of the radio waves from the frequency of oscillation of the electric current, as these frequencies were equal He was able to determine the wavelength of the radio wave by reflecting the waves off a large flat zinc plate to produce a standing wave. A spark in the detector was produced at the anti-nodes of the standing wave, but not at the nodes, so he was able to determine its wavelength As v=fλ, he was able to determine the velocity of the radio wave using the wavelength and pre-determined frequency. He calculated the speed of light to be 3x108ms-1, which was close to Maxwell’s prediction (3.11x108ms-1) and the experimentally measured speed of light Hertz also showed that radio waves could be refracted by passing them through a large asphalt prism. He further showed that they could be polarised, as the detector loop sparked whilst parallel to the detector, but did not when perpendicular to the detector. Thus he showed that radio waves behaved similarly to light waves in terms of velocity, reflection, refraction, interference and polarisation, and concluded that light and radio waves were parts of the broad electromagnetic spectrum Describe Hertz’s observation of the effect of a radio wave on a receiver and the photoelectric effect he produced but failed to investigate During his investigation into the properties of radio waves, Hertz observed that the sparks were easier to produce in the detector loop when it was illuminated with ultraviolet light When he placed a glass panel between the transmitter and receiver, the maximum spark length of the detector loop reduced, as glass absorbs UV light produced by the spark. When he placed quartz there instead, the spark length was unchanged, as quartz does not absorb UV light. He also placed the entire apparatus in a darkened box, and noted that the maximum spark length decreased. His observations were caused by the photoelectric effect, which is the ejection of electrons from the surface of a polished metal surface when light is shone on it. The UV light caused electrons to be ejected to the surface, which made it easier for them to jump across the spark gap when subject to the radio waves. Hertz did not pursue investigation of this effect however, nor did he make any attempt at explaining how the phenomenon was brought about. Identify Planck’s hypothesis that radiation emitted and absorbed by the walls of a black body cavity is quantised A black body is an idealised body that absorbs all incident electromagnetic radiation, and reemits the radiation in a spectrum characteristic to its temperature A black body curve of the radiation emitted is shown below: According the laws of thermodynamics in classical physics, as the wavelength of the black body radiation decreased, the radiance would increase to an infinite amount. This was named the ‘ultraviolet catastrophe’ as it could not be reconciled with the laws of conservation of energy, thus an explanation had to be found. To reconcile this problem, Planck proposed that the radiation absorbed and emitted from a black body is not continuous as waves, but is emitted as discrete packets of energy called quanta (now known as photons). The size of each quantum of energy is characteristic of the frequency of light emitted. Identify Einstein’s contribution to quantum theory and its relation to black body radiation Einstein extended the concept of quantised energy to light waves, reasoning that the energy from light waves may be treated as a stream of discontinuous quanta, called photons. He proposed that black body radiation was emitted as discrete quanta of energy, not as continuous streams of energy under the classical wave model, but explained that wave and particle properties of light can coexist He proposed the following properties of photons: o The amount of energy carried by a photon is proportional to its frequency o A photon cannot transfer part of its energy; it can only transfer all of its energy, or none of it. o The intensity of light is proportional to the number of photons Explain the particle model of light in terms of photons with a particular energy and frequency Many properties of light are best explained if light is considered to consist of a stream of massless particles, or discrete bundles of energy, called photons The energy of a photon is proportional to its frequency => the shorter the wavelength of a photon, the higher the frequency, thus the greater the total energy The intensity of light is the number of photons per unit area, regardless of the energy of each photon All photons travel at the speed of light (3x108ms-1) in a vacuum The success of the particle model of light can be seen when considering the photoelectric effect PHOTOELECTRIC EFFECT AND PHOTONS Consider the apparatus below As explained above, electrons are released from the polished surface of the cathode when light is shone at it due to the photoelectric effect. When electrons are released, they complete the circuit, thus a reading on the voltmeter and ammeter can be read. Many aspects of the photoelectric effect were inconsistent with the classical wave model of light: o No current was registered below a certain frequency of incident light on the cathode. This contrasted to classical theory, which predicted electrons can gain enough energy given enough time from any frequency of EMR to complete the circuit. The cut-off frequency (also known as threshold frequency) depended on the material of the cathode. o The kinetic energy of the electrons increases as the frequency of incident light increases, but there is no change if frequency is constant and intensity is increased. Under the classical wave model, an increase in intensity should increase the energy of the electrons o There is no time delay between the time the light is shone on the surface and the time the electrons are emitted, regardless of the intensity of light. Under the classical model, electrons would require a length of time to gain enough energy from a low-intensity light source in order to escape the surface of the metal Einstein was able to explain the above observations using the fact that a photon’s energy is proportional to its frequency (E=hf, h is constant), and a photon can only transfer all of its energy or none of it. The energy of a photon is transferred entirely to the electron. The minimum energy required to remove an electron from the surface of a metal is called the work function (W), and depends on the type of metal. The kinetic energy of a photoelectron can thus be expressed as the following: From that, we can see that below a certain frequency depending on the work function, kinetic energy will be a negative value, thus no current will flow => this explains the observation of a cut-off frequency As the work function is constant for a particular metal, the final kinetic energy of an electron depends on the frequency of incident light, regardless of its intensity. Thus the particle model of light successfully explains the above observations that were inconsistent with the classical wave model Identify the relationships between photon energy, frequency, speed of light and wavelength: and As stated above, the frequency of light is proportional to its frequency. With the constant of proportionality h, called Planck’s constant, we get: Where: o E = Energy of photon [J] o H = Planck’s constant = 6.626x10-34Js o f = Frequency [Hz] Recall that the velocity of a wave is given by v=fλ. As the speed of photons is constant, the following relationship holds: Where: o c = Speed of light = 3.00x108ms-1 o f = Frequency [Hz} o λ = Wavelength [m] Solve problems and analyse information using: and Remember to always use the correct units before using the above equations. The energy of electrons is commonly given in electron volts (eV), and the wavelength of EMR is often given in nanometres (nm). The conversion from electron volts to joules is given in the data sheet If given data on the kinetic energy of photoelectrons and incident light and asked to calculate frequency, use the wavelength in calculations, as the work function generally needs be taken into account when using the energy of photoelectrons Identify data sources, gather, process and analyse information and use available evidence to assess Einstein’s contribution to quantum theory and its relation to black body radiation Einstein’s quantisation of the model of light allowed for the explanation of the photoelectric effect, particularly the ‘all-or-nothing principle and E=hf. This also explained the work function of metals (the minimum energy a photoelectron requires to escape the surface of a metal) and how this was related to the threshold frequency Using the particle model of light, Einstein was able to successfully explain the why black body radiation intensity reached a peak and then continued to decrease, rather than increase to infinity as predicted by classical physics Thus Einstein’s contribution to quantum theory, in explaining the photoelectric effect and the quantum nature of light and black body radiation, expanded our understanding of quantum mechanics, and opened up the world of modern physics Identify data sources, gather, process and present information to summarise the use of the photoelectric effect in photocells A photocell is a device that converts energy from sunlight into electrical energy, such as solar/photovoltaic cells or photoconductive tubes. The operation of solar/photovoltaic cells will be covered in the next section. The diagram below shows the structure of a phototube The surface of the cathode is made of a high-resistance semiconductor material, such as CdS, PbS and PbTe. Light shines upon the cathode, which causes the material to release electrons from the structure into the conduction band due to the photoelectric effect. These free electrons can conduct electricity quite easily from the cathode to the anode, which significantly reduces the resistance of the material. As a result, a current starts to flow in the circuit, which can trigger another functional system. Photocells can be used as switches that turn on and off depending on the amount of light available. For example: o Street lights can be programmed to turn on when there is less natural light. o Sensors in automatic doors emit infra-red light, which is reflected from an approaching object and triggers the photocell to control opening the doors Photocells are also used in light meters, which measure the amount of light present. An increase in the intensity of light increases the number of photons above the threshold frequency, which increases the number of photoelectrons. The reading on a galvanometer connected to the phototube can be interpreted as a reading of light intensity. Process information to discuss Einstein’s and Planck’s differing views about whether science research is removed from social and political forces Both Planck and Einstein lived in Germany during the early 20th century, and their work was subject to social and political forces under the Nazi regime Planck remained in Berlin to continue research into physics, as he supported the German cause. Planck worked for the war effort in Germany, even though his teachings were sometimes ridiculed. On the other hand, Einstein was a pacifist, and believed the science should be completely removed from social and political forces. He emigrated to the U.S. due to the increasing political discrimination of Jews and the ‘Jewish science’ of relativity. He was prompted by the Nazi atrocities however to encourage Roosevelt to pursue the development of the nuclear bomb despite his pacifist values, which was a decision he later regretted. Perform an investigation to demonstrate the production and reception of radio waves METHOD A DC induction coil was attached to a power supply, and adjusted to give a strong spark between the electrodes (around 2cm). A radio was set to a static frequency and placed 50cm from the induction coil. The induction coil was turned on and off repeatedly, and any observed sounds were noted. The receiver was then placed in different areas of the room no further than 5m away, and three different static frequencies were tested. SAFETY: The sparks produced emit X-rays, which are potentially dangerous for prolonged exposure. Stand at least 2m away from the coil whilst it is turned on (consider the inverse square law), and only turn the coil on for short periods of time (no more than 5 seconds) for observation The sparks also produce ozone, which can aggravate respiratory problems => conduct the experiment in a well-ventilated area, and again only turn the induction coil on for short periods of time RESULTS When the induction coil was turned on, the volume of the static crackling increased significantly, and returned to its normal volume when the coil was turned off The further away the radio was from the induction coil, the smaller the change in observed sound The increase in sound was more pronounced for lower frequencies The crackling sound heard was due to interference of the radio waves produced by the induction coil being received by the radio’s antenna ACCURACY/RELIABILITY/VALIDITY Only qualitative observations were recorded, so accuracy is irrelevant The induction coil was turned on and off several times and produced the same results A control was used, as the radio was kept on whilst the induction coil was turned off, thus the increased static sound can be attributed to the production of radio waves by the induction coil Repeating the procedure with different radio frequencies showed the production of a range of radio waves 3. Limitations of past technologies and increased research into the structure of the atom resulted in the invention of transistors Identify that some electrons in solids are shared between atoms and move freely In some solids, the outer electrons (also known as the valence electrons) are very loosely bound to particular atoms Solid metals exist as a lattice structure containing an orderly array of positive metal ions. Valence electrons are delocalised and free to move throughout the lattice to maintain stability These electrons can move freely across the entirety of the solid in a random pattern. If an electric field is applied across the solid, the electrons move single direction, thus causing electricity to flow In insulators however, the atoms are held together by covalent bonds, which means that the molecules have full valence shells. No electrons are free to move, so these solids are poor conductors of electricity Describe the difference between conductors, insulators and semiconductors in terms of band structures and relative electrical resistance In an atom, negatively-charged electrons orbit a positively-charged nucleus in orbitals which have defined energy (hence the angular momentum of electrons is quantised) In isolated atoms (such as gases), the energy levels for each corresponding orbital of that element are the same According to Pauli’s exclusion principle, no two electrons in the same atom may share the same quantum state, such as orbital energy. Thus if two atoms are brought closely enough together, their electron’s energy levels split slightly so that they have different orbital energies The splitting of energy levels results in two distinct levels close to one another. In solids, billions of atoms are close together (such as in crystalline solids), the energy levels of atoms form continuous but discrete bands rather than discrete value As the number of discrete energy levels becomes large, the gap between the allowed energy bands becomes extremely small. There remain some sections of energy level in which no orbital energy exists: these are called band gaps or forbidden energy levels Two energy bands of particular concern are the valence band and the conduction band o The valence band is the highest energy level occupied by electrons in an atom or molecule in its ground state o The conduction band is the energy level occupied by electrons that are free to move and conduct electricity The difference between the conductivity of conductors, insulators, and semiconductors lies in the energy difference between the valence and conduction bands o In a conductor, the valence band is only partially filled, and the valence and conduction bands overlap. Thus valence electrons are free to move throughout the solid, and allow current to flow when a potential difference is applied across them o In an insulator, the valence band is completely filled, and gap between the valence and conduction band is very large. Valence electrons are resistant to motion when the solid is subjected to an electric field, thus the electrical resistance of insulators are very high. o In a semiconductor (such as silicon or germanium), the gap between the valence and conduction bands is not too large. Under certain conditions, such as external heat or light, electrons in the valence band can gain sufficient energy to cross the gap and enter the conduction band. Thus in semiconductors, electrical resistance decreases markedly with increasing temperature Property Valence band Conduction band Insulators Completely filled Empty Forbidden gap Resistance Very large High Semiconductors Almost filled Empty at absolute zero, partially occupied at high temperatures Small Reduced, particularly at high temperatures Conductors Partly filled Overlaps with the valence band, so electrons are free to move between them None Very low Compare qualitatively the relative number of free electrons that can drift from atom to atom in conductors, semiconductors and insulators Insulators have very few electrons in the conduction band under normal conditions, so the resistance of insulators is very high Conductors have many electrons in the conduction band, so they easily conduct electricity Semiconductors have few electrons in the conduction band, though the number increases with the supply of heat or light energy Identify absences of electrons in a nearly full band as holes, and recognise that both electrons and holes help to carry current When an electron is excited to the conduction band, it leaves a vacancy in the valence band. The absence of an electron in the valence band is called a hole. When a solid is conducting electricity, other electrons can jump from the valence band of neighbouring atoms to fill a hole, subsequently creating a hole in their original position As the temperature of a semiconductor increases, the number of electrons excited to the conduction band increases, thus the number of holes increases The movement of holes can be considered the flow of positive charges, and they move in the opposite direction to electrons when a material is conducting electricity. Thus both electrons and holes help to carry current. Describe how ‘doping’ a semiconductor can change its electrical properties Silicon and germanium are called intrinsic (pure) semiconductors, as their semiconducting properties occur naturally due to the 4 electrons in the valence shell. Doping involves adding specific impurity atoms (dopants) to a semiconductor to change its semiconducting properties. Doped semiconductors are also known as extrinsic semiconductors If the dopant atom has a different number of valence electrons from the atom of the semiconductor, the number of free electrons or holes changes, and hence electrical conductivity is increased Identify differences in p and n-type semiconductors in terms of the relative number of negative charge carriers and positive holes p-type conductors are formed when an element with three electrons in the valence band (Group 3 elements) such as boron or gallium is substituted into the lattice of a semiconductor through doping. Group 4 elements, such as silicon or germanium, have 4 valence electrons, thus four electrons are required to bond with these atoms. The Group 3 atom has only three valence electrons, so a hole is incorporated into the crystal lattice as it is one electron short per dopant atom. Electrons from the valence shells of neighbouring atoms can jump into the hole, causing another hole, and the subsequent movement of the hole through the semiconductor, hence improving electrical conductivity Dopants that produce a charge vacancy or a hole are called acceptor impurities The diagram below shows the lattice of a p-type semiconductor n-type semiconductors are formed when a Group 5 impurity atom (such as phosphorous or arsenic) is substituted into the lattice of the semiconductor Group 5 atoms have 5 valence electrons, but it can only bond with 4 atoms in the silicon crystal lattice. Thus one electron of the impurity is not strongly bonded to the dopant, and can easily be moved into the conduction band, which improves electrical conductivity by lowering the energy required for valence charges to jump into the conduction band Impurities that produce unbonded electrons are called donor impurities. The diagram below shows the lattice of an n-type semiconductor Describe differences between solid state and thermionic devices and discuss why solid state devices replaced thermionic devices Many electrical appliances need to control the direction of current flow, rectify AC into DC, switch current flow on or off, or amplify current Early electronics used thermionic devices to control current flow. Thermionic devices rely on the thermionic emission of electrons from heated filaments and terminals set in glass vacuum tubes Below is a picture of a thermionic diode The heated filament is connected to the external circuit, and only allows current to flow from the cathode to the anode In a thermionic triode, an electrode grid is added to the vacuum diode, which amplifies the current Solid state devices on the other hand use semiconductor materials to control current flow Below is an example of a solid state diode, which operates using the junction between a ptype and an n-type semiconductor The extra free electrons in the n-type semiconductor move across the junction to fill the extra holes in the p-type semiconductor The zone adjacent to the junction (the depletion zone) resists the movement of any more electrons or holes due to the build-up of electrostatic charge, which effectively means that current can flow in one direction only Whilst thermionic and solid state devices both are able to rectify, amplify and switch current, solid state devices have replaced thermionic devices for the following reasons: o Thermionic devices produce large quantities of heat, which can damage the surrounding electronics o Thermionic devices are require separate heating circuits to heat the cathode, which takes time to operate. Solid state devices are more than 100 000 faster to operate and load o High voltages are required to correctly bias thermionic diodes and triodes, whilst silicon transistors use 0.6V to achieve the same effect o Thermionic devices consume much more power than solid state devices and are more expensive to produce, so are much less economical to operate o Thermionic devices are bulky, so they cannot be used in portable devices o The vacuum tube and heating circuits in thermionic devices means that they are very fragile and have very short lifetime Gather, process and present secondary information to discuss how shortcomings in available communication technology lead to an increased knowledge of the properties of materials with particular reference to the invention of the transistor Many communication technologies require the amplification of electrical signals, such as radios, telephones and televisions. Early communication technologies used thermionic devices such as the triode to amplify signals. The use of thermionic devices in communication had many problems however, as they were bulky, consumed a lot of power, had limited response times, and were very fragile. As such, thermionic devices had short lifetimes and were uneconomical, particularly with the growth of telephone networks during the 1940s and 1950s The shortcomings of thermionic devices led scientists to research into solid state replacements that were compact, power-efficient, mechanically durable, and able to operate high switching speeds => this led to the development of the transistor The transistor consists of either a p-n-p junction or an n-p-n junction. An n-p-n transistor is shown below Electrons initially flow from the collector and emitter to the base. If a potential difference is applied across the base and across the emitter, a small current flows from the base and a large current flows from the emitter, thus the transistor amplifies the electrical signal Transistors can also be used to switch current on and off, which allows it to replicate a digital code as an electrical signal The invention of the transistor led to increased research into materials that could be used in transistors, leading to a greater understanding of the properties of semiconductors such as silicon and germanium With further understanding of semiconductors, doping was invented, increasing the versatility of transistors and the scope of applications for which they can be used Identify that the use of germanium in early transistors is related to a lack of ability to produce other materials of suitable purity Both germanium and silicon are group 4 elements, thus were considered suitable in the development of solid state devices using semiconductors Germanium was used initially because the industrial techniques were available at the time to purify germanium were not available for other semiconducting materials such as silicon Once the technology to purify silicon to required level became available, silicon largely replaced germanium transistors Silicon is preferred over germanium because: o Germanium becomes a relatively good conductor when it heats up, which can damage electronic equipment by allowing too much current to flow, which can cause transistors to fail o Silicon is more abundant in Earth’s crust and thus cheaper to produce o Silicon can curry a higher current for longer periods of time Identify data sources, gather, process, analyse information and use available evidence to assess the impact of the invention of transistors on society with particular reference to their use in microchips and microprocessors The invention of the transistor led to the development of integrated circuits, which are tiny electronic circuits used to perform a specific electronic function Modern integrated circuits have millions of circuit elements on a centimetre square of silicon, allowing for smaller microchips to be developed The use of microchips and microprocessors in everyday devices such as cars, games, communications technology, and music players have significantly changed the way people live IMPACT OF TRANSISTORS ON SOCIETY Electronic devices have become much cheaper and more commonplace, allowing more people to access labour-saving and recreational electronic devices Communication is much faster, easier, and cheaper through electronic technologies such as mobile phones and the Internet The development of the Internet and word processing has changed the way people interact and obtain information Credit and debit cards have changed the way people spend money Computerised medical technology has increased life expectancy, but life has become more sedentary due to the widespread use of labour-saving technology Reductions in unskilled jobs due to the computerisation of industry and manufacturing has led to a growth in unemployment Identify data sources, gather, process and present information to summarise the effect of light on semiconductors in solar cells Solar cells use a p-n junction to convert light energy directly into electrical energy Below is a diagram of a typical solar cell The region around the n-type and p-type junction is called the depletion zone. The donor electrons from the n-type semiconductor migrate to the p-type conductor, and holes migrate in the opposite direction. The migration of charges produces an electric field that resists further migration of charges When a photon of light is incident on the p-type semiconductor causes electrons to be excited to the conduction band. These electrons move across the junction to the n-type semiconductor due to the electric field, and holes move in the opposite direction These electrons have a much higher lifetime before recombining with a hole. Electrodes placed on the ends of the semiconductor collect the current, which provides electrical power as the electrons return to the p-type conductor via an external circuit Solar cells are manufactured to be thin enough so that most of the incoming light is transmitted to the p-type semiconductor. An anti-reflection coating on the surface also helps to maximise the number of electrons that are excited by incident photons. Perform an investigation to model the behaviour of semiconductors, including the creation of a hole or positive charge on the atom that has lost the electron and the movement of electrons and holes in opposite directions when an electric field is applied across the semiconductor METHOD Ten marbles were placed in a row on a table, and two random marbles were removed. The remaining marbles were then moved towards the left by sequentially moving a marble adjacent to a hole Use a diagram to show the marbles (electrons) and gaps (holes) resting on the table (valence band) and lifted (conduction band) RESULTS The original ten marbles resting on the table represent the electrons in the valence band of a semiconductor Removing the two marbles represents the excitation of electrons to the conduction band, and the gap left by the marbles represent holes or positive charges The movement of electrons to the left represents the movement of electrons in the opposite direction to an electric field. Both the marbles and the holes were observed to move in opposite directions NOTE: If asked a question on this practical, make sure to show how holes/electrons are able to move with energy input across the forbidden energy level from the valence band to the conduction band, and that both electrons and holes are able to move, but in opposite directions ACCURACY/RELAIBLITY/VALIDITY As this was a qualitative model of semiconductors, accuracy and reliability are irrelevant Validity was established as the model was carefully thought out, and symbols corresponded to physical principles The validity of the model was undermined by the oversimplification of conduction in semiconductors 4. Investigations into the electrical properties of particular metals at different temperatures led to the identification of superconductivity and the exploration of possible applications Outline the methods used by the Braggs to determine crystal structure Sir William and Lawrence Bragg studied the structure of crystals analysing the interference pattern of scattered X-rays produced from the diffraction of rays incident on a crystalline solid The X-rays were produced from cathode rays hitting a metal anode and directed at a metal salt in a 40kV X-ray tube. These rays were collimated so they remained parallel upon reflection from the crystalline solid The diagram below shows parallel X-rays striking a crystal. Note that whilst X-rays are scattered, the model below uses reflection to simplify the description Bragg’s equation can be used to calculate the distance between the planes: The Braggs used an ionisation chamber to determine the position and intensity of the diffracted X-rays, and photographic film to provide an image of the interference pattern Using mathematical analysis and calculations based on the spacing between maxima in the X-ray interference pattern , the Braggs were able to confirm the crystal lattice of materials – parallel layers of orderly arranged atoms Identify that metals possess a crystal lattice structure Metals are often described as a crystal lattice of positive ions surrounded by a cloud of delocalised electrons Metals are considered crystalline as the structure is uniform and not amorphous (i.e. no distinct shape) Some examples of the structure of metallic crystals are shown below (body-centred cubic, face-centred cubic and hexagonal close-packed) Describe conduction in metals as a free movement of electrons unimpeded by the lattice As mentioned above, metallic crystals contain a cloud of delocalised electrons that move in random directions throughout the lattice When an electric field is applied, the delocalised electrons experience a force; hence electrons experience a net movement in one direction when conducting electricity As the electrons are delocalised, their movement during conduction is generally unimpeded by the lattice Identify that resistance in metals is increased by the presence of impurities and scattering of electrons by lattice vibrations Electrons generally experience resistance when conducting, as the kinetic energy of the electrons is converted to heat and other forms of energy Recall from the preliminary course that resistance depends on the following factors: o Cross-sectional area o Temperature o Length of conductor o Resistivity of conducting material The temperature of a metal can be considered as a measure of the vibration of lattice ions due to their energy => the more the ions vibrate, the higher the temperature The lattice vibrations cause electrons to scatter as they collide with the lattice ions. The scattering of electrons decreases their kinetic energy and increases resistance of the conductor. The presence of impurities and imperfections in the conductor also causes obstructions to electron movement and increase resistance, as they are not necessarily part of the lattice Describe the occurrence in superconductors below their critical temperature of a population of electron pairs unaffected by electrical resistance Superconductivity is the phenomenon in which a conductor exhibits negligible resistance when cooled past its critical temperature (Tc) The graph below shows the resistance of a superconductor and a normal conductor as a function of temperature As mentioned above, lattice vibrations of a conductor inhibit the movement of electrons, which increases resistance On the other hand, below the critical temperature of superconductors, the lattice actually assists electrons flow, and electron pairs are unaffected by electrical resistance (see below for further detail) There are two types of semiconductors, type I and type II o Type I superconductors are pure metals, and have a critical temperature of up to 23K o Type II semiconductors are ceramics and metal alloys, and have a critical temperature of up to 120K Process information to identify some of the metals, metal alloys and compounds that have been identified as exhibiting the property of superconductivity and their critical temperatures Element/alloy/compound Aluminium Tin Mercury Lead Technetium Niobium-nitride Vanadium-silicon Tin-Niobium alloy Ni-Al-Ge Alloy MgB2 Alkali-doped fullerene (RbCs2C60) YBa2Cu3O7 (YBCO) Bi2Sr2Ca2Cu3O10 HgBa2Ca2Cu3O8 Tc (K) 1.2 3.73 4.12 7.22 11.2 16 17.5 18 21 39 33 92 110 133 Tc (°C) -271.95 -269.42 -269.03 -265.93 -261.95 -257.15 -255.65 -255.15 -252.15 -234.15 -240.15 -181.15 -163.15 -140.15 Discuss the BCS theory BCS theory (named after physicists Bardeen, Cooper, and Schrieffer) is used to explain superconductivity in type I superconductors, but cannot explain its occurrence in type II superconductors According to BCS theory, when a conductor is cooled to below its critical temperature, the very low thermal energy (i.e. reduced random lattice vibrations) leads to a quantum state where an electron can lead to a distortion in the lattice of positive ions This distortion, also called a phonon, gives rise to a localised positive region, which attracts a second electron with opposite spin in to combine with the first as a ‘Cooper pair’. The electron pairing is favoured as it puts electrons into a lower energy state The Cooper pair interacts with the lattice to produce destructive interference with the distortion, so the phonon’s lifetime is too short to propagate through the lattice like a wave. As a result, electrons are able to move through the lattice with no loss of energy, hence a superconductor in its superconducting state has no electrical resistance The diagrams below show the occurrence of phonons in superconductors BCS Theory was highly successful in explaining the microscopic and macroscopic properties of some semiconductors. It predicted certain properties that were verified later, such as the Meissner effect and heat capacity On the other hand, BCS Theory cannot explain the occurrence of high-temperature superconductivity in ceramics Analyse information to explain why a magnet is able to hover above a superconducting material that has reached the temperature at which it is superconducting Below its critical temperature, a superconductor becomes perfectly diamagnetic and cancels all magnetic flux inside => this is called the Meissner effect Consider the diagram below of a superconductor in its normal state and superconducting state An external magnetic field is able to penetrate the superconductor in its normal state, but produces currents in its superconducting state, which cancel all magnetic flux inside the superconductor When a magnet is brought near a superconductor in its superconducting state, the induced eddy currents produce an image of the permanent magnet to cancel all magnetic flux within the superconductor. This results in a repulsion that balances the weight of the magnet, thus causing levitation Discuss the advantages of using superconductors and identify limitations to their use ADVANTAGES The main advantage of superconductors is that there is no heat lost when passing current through them, which significantly reduces power losses in the transmission of electricity and allows induced currents to persist indefinitely. o This is particularly useful in electromagnets, as its magnetic field persists after the power supply has been turned off Superconducting electromagnets are also amongst the most powerful magnets known, which is useful in maglev trains and medical diagnostic equipment (e.g. MRI) LIMITATIONS The main disadvantage of superconductors is the very low temperatures required to maintain their superconducting state, which significantly reduces their practicality o Metals require liquid helium to reach their superconducting state, whilst hightemperature ceramic superconductors require liquid nitrogen Superconductors are also very expensive due to the required low temperatures High-temperature ceramic superconductors are too brittle to be shaped easily, thus have limited useful applications Superconductors are also very sensitive to moving magnetic fields, meaning applications using AC (e.g. transistors) may need research in order to use DC instead Process information to discuss possible applications of superconductivity and the effects of those applications on computers, generators and motors and transmission of electricity through power grids COMPUTERS The speed and further miniaturisation of computer chips are limited by the generation of heat, and the speed with which signals can be conducted => these two factors could be improved by using superconductors in electronics One application of superconductors in electronics is the Josephson junction, which consists of two superconductors separated by an oxide layer, and acts as a superfast switch By using superconducting film such as the Josephson junction, integrated circuits could be more densely packed due to the reduction in heat generated => this would allow for far greater computing power and more energy-efficient electronics As superconductors have no resistance in their superconducting state, electrical signals can also be transmitted faster, allowing information to be processed several orders of magnitude faster GENERATORS AND MOTORS The electromagnets used in large-scale generators heat up significantly due to the resistance of their wires. Electromagnets also require an iron core to strengthen the field, which increases power required to rotate the magnet due to its heavy mass Using superconducting electromagnets without iron cores could potentially halve the amount of power lost in the electromagnets, which would significantly increase the power output of generators Superconducting magnets in motors provide a much stronger magnetic field than permanent magnets, so no iron core would be needed => this would lower the weight of the rotor and increase energy-efficiency TRANSMISSION OF ELECTRICITY THROUGH POWER GRIDS Recall that electricity is transmitted at low currents to minimise power losses in wires (P=I2R) The lack of resistance in superconductors means that electricity could be transmitted without loss of energy as heat. This would allow them to carry many times more current than conventional wires. Superconducting transmission wires would also be able to transmit electricity in DC, as voltage would not need to be stepped-up in transformers. This would minimise power losses in transmission, as the production of eddy currents is a major source of energy loss in AC transmission, particularly in transformers NOTE: Remember to consider the impact of each application on society and the environment. Generally, energy-efficient = reduced cost (society) = less dependence on fossil fuels (environment) Gather and process information to describe how superconductors and the effects of magnetic fields have been applied to develop the maglev train Magnetic levitation (maglev) trains have been built that use powerful electromagnets made from superconductors Maglev trains require extremely powerful magnets as they are very heavy, so only superconducting magnets can be used The diagrams below show the basic structure of maglev trains The superconducting magnets are mounted on the train and kept cool with liquid helium Once the train is levitated using magnetic repulsion from powerful superconductor magnets, the polarity of alternate magnets on the track is continually changed, which generates a series of attractions and repulsions to accelerate the train The superconducting electromagnets rely on conventional ‘like-pole’ repulsion to achieve levitation, not the Meissner effect BENEFITS Magnetic levitation provide a frictionless method of movement as the train is free from any surface, which allows for high speeds to be achieved Maglev trains are also quieter than conventional train system, despite reaching much higher speeds LIMITATIONS Extremely large amounts of energy are expended, which impedes wider use due to the negative economic and environmental impact The electromagnet that levitate the train are expensive to run over long distances, and the new lines that have to be constructed also increase the cost of implementing maglev trains on a wider scale The strong magnetic field inside the train excludes passengers with pacemakers, and may damage devices that use magnetic storage such as computers and credit cards Perform an investigation to demonstrate magnetic levitation METHOD A high-temperature superconductor disc was placed in a Styrofoam cup and immersed with liquid nitrogen, allowing the disc to cool to below its critical temperature. A neodymium magnet was then carefully placed, using plastic tweezers, just above the superconducting disc SAFETY Liquid nitrogen can cause severe damage to skin due to its very low temperature => only the teacher should handle the liquid nitrogen, using safety glasses, cotton gloves and plastic tweezers to handle the magnet and the superconductor in the Styrofoam cup, which is an insulator RESULTS The neodymium magnet hovered above the disc for about five minutes, then slowly came to rest on top of the disc as the liquid nitrogen disappeared See above for an explanation of magnetic levitation and the Meissner effect ACCURACY/RELIABILITY/VALIDITY The experiment was qualitative, so accuracy is irrelevant Magnetic levitation was demonstrated multiple times, so the experiment was reliable All the conditions for magnetic levitation were met, such as using a superconductor below it critical temperature and a neodymium magnet, so the experiment was valid