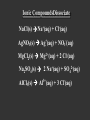* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ELECTROLYTES: NONELECTROLYTES
Ionic liquid wikipedia , lookup
Electrochemistry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
History of electrochemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Ionic compound wikipedia , lookup
Electrolysis of water wikipedia , lookup
Ch. 4 Types of Chemical Reactions and Solution Stoichiometry Solute A solute is the dissolved substance in a solution. Salt in salt water Sugar in soda drinks Carbon dioxide in soda drinks Solvent A solvent is the dissolving medium in a solution. Water in salt water Water in soda Saturation of Solutions A solution that contains the maximum amount of solute that may be dissolved under existing conditions is saturated. A solution that contains less solute than a saturated solution under existing conditions is unsaturated. A solution that contains more dissolved solute than a saturated solution under the same conditions is supersaturated. Definition of Electrolytes and Nonelectrolytes An electrolyte is: A substance whose aqueous solution conducts an electric current. A nonelectrolyte is: A substance whose aqueous solution does not conduct an electric current. Try to classify the following substances as electrolytes or nonelectrolytes… Electrolytes? 1. 2. 3. 4. 5. 6. 7. Pure water Tap water Sugar solution Sodium chloride solution Hydrochloric acid solution Ethyl alcohol solution Pure, solid sodium chloride Answers… ELECTROLYTES: NONELECTROLYTES: Tap water (weak) Pure water NaCl solution Sugar solution HCl solution Ethanol solution Pure, solid NaCl But why do some compounds conduct electricity in solution while others do not…? Ionic CompoundsDissociate NaCl(s) Na+(aq) + Cl-(aq) AgNO3(s) Ag+(aq) + NO3-(aq) MgCl2(s) Mg2+(aq) + 2 Cl-(aq) Na2SO4(s) 2 Na+(aq) + SO42-(aq) AlCl3(s) Al3+(aq) + 3 Cl-(aq) Ions tend to stay in solution where they can conduct a current rather than re-forming a solid. The reason for this is the polar nature of the water molecule… Positive ions associate with the negative end of the water dipole (oxygen). Negative ions associate with the positive end of the water dipole (hydrogen). Some covalent compounds IONIZE in solution Covalent acids form ions in solution, with the help of the water molecules. For instance, hydrogen chloride molecules, which are polar, give up their hydrogens to water, forming chloride ions (Cl-) and hydronium ions (H3O+). Strong acids such as HCl are completely 100% ionized in solution. Other examples of strong acids include: Sulfuric acid, H2SO4 Nitric acid, HNO3 Hydriodic acid, HI Perchloric acid, HClO4 Weak acids such as lactic acid usually ionize less than 5% of the time. Many of these weaker acids are “organic” acids that contain a “carboxyl” group. The carboxyl group does not easily give up its hydrogen. Because of the carboxyl group, organic acids are sometimes called “carboxylic acids”. Other organic acids and their sources include: o Citric acid – citrus fruit o Malic acid – apples o Butyric acid – rancid butter o Amino acids – protein o Nucleic acids – DNA and RNA o Ascorbic acid – Vitamin C This is an enormous group of compounds; these are only a few examples. However, most covalent compounds do not ionize at all in solution. Sugar (sucrose – C12H22O11), and ethanol (ethyl alcohol – C2H5OH) do not ionize - That is why they are nonelectrolytes! Molarity The concentration of a solution measured in moles of solute per liter of solution. M = mol L Preparation of Molar Solutions Problem: How many grams of sodium chloride are needed to prepare 1.50 liters of 0.500 M NaCl solution? Step #1: Ask “How Much?” (What volume to prepare?) Step #2: Ask “How Strong?” (What molarity?) Step #3: Ask “What does it weigh?” (Molar mass is?) 1.500 L 0.500 mol 1L 58.44 g 1 mol = 43.8 g Serial Dilution It is not practical to keep solutions of many different concentrations on hand, so chemists prepare more dilute solutions from a more concentrated “stock” solution. Problem: What volume of stock (11.6 M) hydrochloric acid is needed to prepare 250. mL of 3.0 M HCl solution? MstockVstock = MdiluteVdilute (11.6 M)(x Liters) = (3.0 M)(0.250 Liters) x Liters = (3.0 M)(0.250 Liters) 11.6 M = 0.065 L A. Single Replacement Reactions A + BX AX + B BX + Y BY + X Replacement of: Metals by another metal Hydrogen in an acid by a metal Hydrogen in water by a metal Halogens by more active halogens Ex. Mg(s) + HCl(aq) MgCl2(aq) + H2(g) Ex. 2 Li(s) + 2 H2O(l) 2 LiOH(aq) + H2(g) The Activity Series of the Metals Lithium Potassium Calcium Sodium Magnesium Aluminum Zinc Chromium Iron Nickel Lead Hydrogen Bismuth Copper Mercury Silver Platinum Gold Metals can replace other metals provided that they are above the metal that they are trying to replace. Metals above hydrogen can replace hydrogen in acids. Metals from sodium upward can replace hydrogen in water The Activity Series of the Halogens Fluorine Chlorine Bromine Iodine Halogens can replace other halogens in compounds, provided that they are above the halogen that they are trying to replace. 2NaCl(s) + F2(g) 2NaF(s) ??? + Cl2(g) MgCl2(s) + Br2(g) No Reaction ??? Practice problems - Answers are unbalanced! 1. Mg + FeCl3 Fe + MgCl2 2. Sodium is added to water. Na + H2O H2 + NaOH 3. Lithium is added to hydrochloric acid Li + HCl H2 + LiCl 4. Zinc is added to a solution of sodium chloride Zn + NaCl N.R. 5. Chlorine gas is bubbled into a solution of potassium iodide Cl2 + KI I2 + KCl 6. Chlorine gas is bubbled into a solution of potassium fluoride Cl2 + KF N. R. Double Replacement Reactions The ions of two compounds exchange places in an aqueous solution to form two new compounds. AX + BY AY + BX One of the compounds formed is usually a precipitate (an insoluble solid), an insoluble gas that bubbles out of solution, or a molecular compound, usually water. Double replacement forming a precipitate… Double replacement (ionic) equation Pb(NO3)2(aq) + 2KI(aq) PbI2(s) + 2KNO3(aq) Complete ionic equation shows compounds as aqueous ions Pb2+(aq) + 2 NO3-(aq) + 2 K+(aq) +2 I-(aq) PbI2(s) + 2K+(aq) + 2 NO3-(aq) Net ionic equation eliminates the spectator ions Pb2+(aq) + 2 I-(aq) PbI2(s) Solubility Rules – Mostly Soluble Ion NO3- Solubility Soluble Exceptions None ClO4- Soluble None Alkali metals NH4+ Soluble None Soluble None Cl-,Br-, I- Soluble Pb2+, Ag+, Hg22+ SO42- Soluble Ca2+, Ba2+, Sr2+, Pb2+, Ag+, Hg2+ C2H3O2- Soluble Ag+ Solubility Rules – Mostly Insoluble Ion Exceptions CO32- Solubility Insoluble PO43- Insoluble Group IA and NH4+ OH- Insoluble Group IA and Ca2+, Ba2+, Sr2+ S2- Insoluble Groups IA, IIA, and NH4+ CrO4-2 Insoluble Group IA & NH4+, Ca2+, Sr2+ SO3-2 Insoluble Group IA and NH4+ Group IA and NH4+ D.R. Practice problems 1. KBr(aq) + AgNO3(aq) AgBr(s) + KNO3(aq) 2. Silver nitrate + potassium chromate 2AgNO3(aq) + K2CrO4(aq) AgCrO4(s) + 2KNO3(aq) 3. Ammonium chloride + cobalt (II) sulfate 2NH4Cl(aq) + CoSO4(aq) (NH4)2SO4(aq) + CoCl2(aq) N.R. 4. Lithium hydroxide + sodium chromate 2LiOH(aq) + Na2CrO4(aq) 2NaOH(aq) + Li2CrO4(s) 5. Zinc acetate + cesium hydroxide Zn(C2H3O2)2(aq) + 2CsOH(aq) Zn(OH)2(s) + 2CsC2H3O2(aq) 6. What is the net ionic equation for the rxn above? Zn+2(aq) + OH-(aq) Zn(OH)2(s) Unstable Compounds!!! (own note paper) • Ammonium hydroxide – NH4OH • Carbonic Acid – H2CO3 • Sulfurous acid – H2SO3 • Sulfide salts (ex. Na2S) from acid (H+) • All break down to form other products! – NH4OH NH3(g) + H2O(l) – H2CO3 CO2(g) + H2O(l) – H2SO3 SO2(g) + H2O(l) – S-2 H2S(g) Unstable Examples: 1. Sodium sulfite + hydrochloric acid Na2SO3(aq) + 2HCl(aq) H2SO3(aq) + 2NaCl(aq) H2SO3(aq) H2O(l) + SO2(g) Na2SO3(aq) + 2HCl(aq) H2O(l) + SO2(g) + 2NaCl(aq) 2. Ammonium sulfate + sodium hydroxide (NH4)2SO4(aq) + NaOH(aq) 2 NH4OH(aq) + Na2SO4(aq) 2 NH4OH(aq) 2 NH3(g) + H2O(l) (NH4)2SO4(aq) + 2NaOH(aq) 2NH3(g) + 2H2O(l) + Na2SO4(aq) What is the net ionic equation for the reaction above? NH4+(aq) + OH-(aq) NH3(g) + H2O(l)