* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunomodulators
Survey
Document related concepts
Transcript
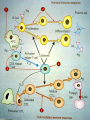
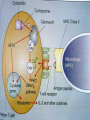
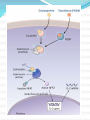
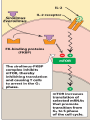
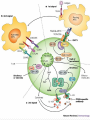
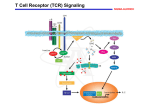
Definition An immunomodulator is a substance(eg. a drug) which has an effect on the immune system. 2 types Immunosuppressants Immunostimulants The Immune Response - why and how ? Discriminate: Self / Non self Destroy: Infectious invaders Dysregulated self (cancers) Immunity: Innate, Natural Adaptive, Learned Mechanisms of immunomodulation Drugs may modulate immune mechanism by either suppressing or by stimulating one or more of the following steps: Antigen recognition and phagocytosis Lymphocyte proliferation/differentiation Synthesis of antibodies Ag –Ab interaction Release of mediators due to immune response Modification of target tissue response The importance of immune system in protecting the body against harmful molecules is well recognized However, in some instances, this protection can result in serious problems E.g, the introduction of allograft can elicit a damaging immune response transplanted tissue causing rejection of the IMMUNOSUPPRESSANTS The importance of the immune system In protecting the body against harmful foreign molecules is well recognized. However, in some instances, this protection can result in serious problems. For example, the introduction of an allograft (that is, the graft of an organ or tissue from one individual to another who is not genetically identical) can elicit a damaging immune response, causing rejection of the transplanted tissue. Transplantation of organs and tissues (for example, kidney, heart, or bone marrow) Has become routine due to improved surgical techniques and better tissue typing. Also, drugs are now available that more selectively inhibit rejection of transplanted tissues while preventing the patient from becoming immunologically compromised. Earlier drugs were nonselective Patients frequently succumbed to infection due to suppression of both the antibody-mediated (humoral) and cell-mediated arms of the immune system. Today, the principal approach to immunosuppressive therapy To alter lymphocyte function using drugs or antibodies against immune proteins. Because of their severe toxicities when used as monotherapy, A combination of immunosuppressive agents, usually at lower doses, is generally employed. Immunosuppressive drug regimens Consist of anywhere from two to four agents With different mechanisms of action that disrupt various levels of T-cell activation. IMMUNE ACTIVATION CASCADE A three-signal model. Signal 1 Constitutes T-cell triggering at the CD3 receptor complex by an antigen on the surface of an antigen-presenting cell (APC). Signal 2, also referred to as costimulation, Occurs when CD80 and CD86 on the surface of apcs engage CD28 on T cells. Both Signals 1 and 2 Activate several intracellular signal transduction pathways, one of which is the calcium calcineurin pathway Which is targeted by cyclosporine and tacrolimus. These pathways trigger the production of cytokines such as interleukin (IL)-2, IL-15, CD154, and CD25. IL-2 then binds to CD25 (also known as the IL-2 receptor) on the surface of other T cells to activate mammalian target of rapamycin (mTOR) Providing Signal 3, the stimulus for T-cell proliferation. IMMUNOSUPPRESSANT DRUGS These categorized according to their MOA: 1. 2. 3. Some agents interfere with cytokine production or action (Calcineurin inhibitors/specific T-cell inhibitors) Others disrupt cell metabolism, preventing lymphocyte proliferation (cytotoxic drugs) Mono, and polyclonal antibodies block T cell surface molecules (antibodies) IMMUNOSUPPRESSANT DRUGS CLASSIFICATION 1. Calcineurin inhibitors (specific T –cell inhibitors): Cyclosporine, Tacrolimus 2. M-TOR inhibitorsSirolimus, Everolimus 3. Antiproliferative drugs (cytotoxic drugs) Azathioprine, cyclophosphamide, methotrexate, chlorambucil, mycophenolate mofetil (MMF) 4. Glucocorticoids Prednisolone and others 5. Biological agents TNFα inhibitors Etanercept, Infliximab, Adalimumab IL-1 receptor antagonist: Anakinra, Rilonacept IL-2 receptor antagonist: Daclizumab, Baciliximab Monoclonal antibodies Anti CD3 antibody: Muromonab Anti CD52 antibody: Alemtuzumab Polyclonal Antibodies: Antithymocyte Antibody (Atg), Rho (D) immuneglobulin. /////////////////////// Philip F. Halloran:N Engl J Med 2004;351:2715-29 Immunosuppressants Organ transplantation Autoimmune diseases Problem Life long use Infection, cancers Nephrotoxicity Diabetogenic SELECTIVE INHIBITORS OF CYTOKINE PRODUCTION AND FUNCTION Cytokines are soluble, antigen-nonspecific, signaling proteins That bind to cell surface receptors on a variety of cells. The term cytokine includes the molecules known as Interleukins (ILs), interferons (IFNs), tumor necrosis factors (TNFs), transforming growth factors, and colony-stimulating factors. IL-2, a growth factor That stimulates the proliferation of antigen-primed (helper) T cells Which subsequently produce more IL-2, IFN-γ, and TNF-a. These cytokines collectively activate Natural killer cells, macrophages, and cytotoxic T lymphocytes. Drugs that interfere with the production or activity of IL-2, Such as cyclosporine, will significantly dampen the immune response and, thereby, decrease graft rejection. CYCLOSPORINE Cyclic peptide composed of 11 aa Extracted from a soil fungus Selectively inhibit T lymphocyte proliferation IL-2 & other cytokine production Response of inducer T cells to IL-1, without any effect on suppressor T cells. Lymphocytes are arrested at G0 or G1 phase MECHANISM OF ACTION Cyclosporine preferentially suppresses cell mediated immune reactions humoral immunity is affected to a far lesser extent. After diffusing into the T cell, Cyclosporine binds to a cyclophilin (called an immunophilin) To form a complex that binds to calcineurin. The latter is responsible for dephosphorylating NFATc (cytosolic Nuclear Factor of Activated T cells). Because the cyclosporine-calcineurin complex cannot perform this reaction, NFATc cannot enter the nucleus To promote the reactions that are required for the synthesis of a number of cytokines, including IL-2. The end result is a decrease in IL-2 Which is the primary chemical stimulus for increasing the number of T lymphocytes. Activated T cells produces IL2 via Dephosphorilation of NAFT (nuclear factor activated T cell - a cytoplasmic transcription factor) Calceneurin is needed for dephosphorilation (Cytoplasmic Phosphatase) Translocates to nucleus Transcriptin of IL2 gene. • Cyclosporine bind s to cyclophilin - binding protein. • Tacrolimus binds to FKBP – FK binding protein. (nuclear factor activated T cell) TOXICITY : CYCLOSPORINE Renal dysfunction Tremor Hirsuitism (abnormal growth of hair on a woman's face and body.) Hypertension Hyperlipidemia Gum hyperplasia (Increase in the size of the gingiva (gums).) Hyperuricemia (abnormally high level of uric acid in the blood)- worsens gout Calcineurin inhibitors + Glucocorticoids = Diabetogenic USES To prevent rejection of kidney, liver, cardiac, BM and other allogeneic transplants. More effective admininistered. when glucocorticoids are also Useful in autoimmune disease as well. Alternative to methotrexate for the treatment of severe, active RA. 2nd line drug for uveitis, bronchial asthma, etc. TACROLIMUS Structure-macrolide like Chemically different immunosuppressant from cyclosporine, newer Macrolide that is isolated from soil fungus Mechanism Similar to cyclosporine except binds to different protein FK- 506 binding protein that inhibits calcineurin. Inhibits synthesis and release of IL-2 CLINICAL APPLICATION Approved for the prevention of rejection of liver and kidney transplants Given with a corticosteroid and/or an antimetabolite. Favor over cyclosporine, Because of Its potency Decreased episodes of rejection Also lower doses of corticosteroids can be used Thus reducing the steroid-associated adverse effects. ADVERSE EFFECTS Nephrotoxicity and neurotoxicity (tremor, seizures, and hallucinations) Development of post transplant, insulin-dependent diabetes mellitus. Other toxicities are the same as those for cyclosporine Except that tacrolimus does not cause hirsutism or gingival hyperplasia. Have a lower incidence of cardiovascular toxicities Such as hypertension and hyperlipidemia Anaphylactoid reactions to the injection vehicle. M-TOR INHIBITORS-SIROLIMUS Structure-macrolide like (Multi-membered lactone ring ) Triene macrolide antibiotic From Streptomyces hygroscopicus MECHANISM OF ACTION Sirolimus and tacrolimus bind to the same cytoplasmic FK binding protein But instead of forming a complex with calcineurin Sirolimus binds to mTOR, interfering with Signal 3. The latter is a serine-threonine kinase. TOR proteins are essential for Many cellular functions, such as cell-cycle progression, DNA repair, and as regulators involved in protein translation. Binding of sirolimus to mTOR Blocks the progression of activated T cells from the G1 to the S phase of the cell cycle and, consequently, the proliferation of these cells. Unlike cyclosporine and tacrolimus, Sirolimus does not owe its effect to lowering IL-2 production but, rather, to inhibiting the cellular responses to IL-2. ADVERSE EFFECTS A common adverse effect Hyperlipidemia (elevated cholesterol and triglycerides) The combination of cyclosporine and sirolimus More nephrotoxic Necessitating lower doses. Other untoward problems are Headache, nausea and diarrhea, leukopenia, and thrombocytopenia. Impaired wound healing in obese patients and those with diabetes. CLINICAL APPLICATION Approved for use in renal transplantation To be used together with cyclosporine and corticosteroids. The combination of sirolimus and cyclosporine Synergistic Because sirolimus works later in the immune activation cascade. The antiproliferative action of sirolimus has found use in cardiology. Sirolimus-coated stents vasculature inserted into the cardiac Inhibit restenosis of the blood vessels by reducing proliferation of the endothelial cells. In addition to its immunosuppressive effects Also inhibits proliferation of cells in the graft intimal areas Thus, is effective in halting graft vascular disease. EVEROLIMUS Another mTOR inhibitor, MECHANISM OF ACTION: Same mechanism of action as sirolimus. It inhibits activation of T cells by forming a complex with FKBP-12 and subsequently blocking mTOR. CLINICAL APPLICATION: Approved for use in renal transplantation. in combination with basiliximab, cyclosporine, and corticosteroids It is also indicated for second-line treatment in patients with advanced renal cell carcinoma. ADVERSE EFFECTS: Adverse effects similar to sirolimus. An additional adverse effect Angioedema kidney arterial and venous thrombosis. azathiopurine Antimetabolite DESCRIPTION Prodrug that releases 6-mercaptopurine Widespread use in organ transplantation MECHANISM Purine synthesis inhibitor Inhibiting the proliferation of cells, especially leukocyte. Converts 6-mercaptopurine to tissue inhibitor of metalloproteinase, which is converted to thioguanine nucleotides that interfere with DNA synthesis; thioguanine derivatives may inhibit purine synthesis Prevents mitosis and proliferation of rapidly dividing cells, such as activated B and T lymphocytes •Azathioprine is metabolized to 6-mercaptopurine (6-MP), which is either inactivated by two enzymes, thiopurine s-methyltransferase (TPMT) and xanthine oxidase (XO), or is further metabolized to thioinosine monophosphate (TIMP). azathiopurine Prescribed for FDA : Renal transplantation Rheumatoid Arthritis Non-FDA : Multiple Sclerosis (disease involving damage to the sheaths of nerve cells in the brain and spinal cord), Crohn's disease (a chronic inflammatory disease of the intestines, especially the colon and ileum) Myasthenia gravis, chronic ulcerative colitis, and autoimmune hepatitis Side effect Leukopenia, bone marrow depression, liver (uncommon); blood-count monitoring required toxicity CYCLOPHOSPHOMIDE More marked effect on B cells and humoral immunity. Used in BM transplantation in which short course with high dose is given. In other organ transplantations it is employed only as a reserve drug. In RA, it is rarely used. Low doses are occasionally employed for maintenance therapy in SLE (autoimmune disease affect the skin, joints, kidneys, brain, and other organs.) and thrombocytopenic purpura. METHOTREXATE Folate antagonist Markedly depresses cytokine production and cellular immunity and has anti-inflammatory property Used as 1st line drug in many autoimmune diseases like rapidly progressing RA, severe psoriasis (a skin disease marked by red, itchy, scaly patches), myasthenia gravis, uveitis, chronic active hepatitis Low dose as maintenance therapy is relatively well tolerated. MYCOPHENOLATE MOFETIL MECHANISM: As an ester, it is rapidly hydrolyzed in the GI tract to mycophenolic acid. Mycophenolic acid is quickly and almost completely absorbed after oral administration. This is a potent, reversible, noncompetitive inhibitor of inosine monophosphate dehydrogenase, which blocks the de novo formation of guanosine phosphate. Thus,like 6-MP, it deprives the rapidly proliferating T and B cells of a key component of nucleic acids. ADR: Most common adverse effects: GI, including diarrhea, nausea, vomiting, and abdominal pain. High doses of mycophenolate mofetil Associated with a higher risk of CMV infection. CLINICAL APPLICATION: Replaced azathioprine because of its safety and effcacy in prolonging graft survival. It has been successfully used in heart, kidney, and liver transplants. GLUCOCORTICOIDS Potent immunosuppressant and antiinflammatory MECHANISM: Inhibits several components of the immune response They particularly (-) MHC expression and proliferation of T lymphocytes. Expression of several IL and other cytokine genes is regulated by corticosteroids. The short lived rapid lymphopenic effect of steroids is due to sequestration of lymphocytes in tissues. Inhibition of gene expression Glucocorticoids Steroid hormones, have broad anti inflammatory activity. Binds with glucocorticoid receptor. glucocorticoid - glucocorticoid receptor complex translocate to nucleus. Binds to GREs in specific genes ( glucocorticoid response elements) Regulates the gene expression down regulate the inflammatory mediators. (IL1, IL4 TNFα etc.) USES - GLUCOCORTICOIDS Transplant rejection BM transplantation Autoimmune diseases – RA, SLE, Hematological conditions Psoriasis Inflammatory Bowel Disease, Eye conditions TOXICITY Growth retardation Avascular Necrosis of Bone Risk of Infection Poor wound healing Cataract Hyperglycemia Hypertension IL-2 RECEPTOR ANTIBODIES BASILIXIMAB MECHANISM:- _ Chimeric murine monoclonal antibody against human IL-2 receptor alpha subunit of activated T to block T cell _ Blocks activation and inhibits clonal expansion of T cells Blockade of this receptor foils the ability of any antigenic stimulus to activate the T-cell response system. GI toxicity as the main adverse effect. USE: _ Used to induce immunosuppression and to prolong organ transplants in combination with immunosuppressants. DACLIZUMAB DESCRIPTION Humanized monoclonal antibody (interleukin-2– receptor α chain) against CD25 MECHANISM Binds to and blocks the interleukin-2– receptor α chain (CD25 antigen) binds with high-affinity to the Tac subunit of the high-affinity IL-2 receptor complex and inhibits IL-2 binding on activated T cells, depleting them and inhibiting interleukin-2–induced T-cell activation DACLIZUMAB PRESCRIBED FOR • Renal, cardiac transplant SIDE EFFECT Hypersensitivity reactions (uncommon) Gastrointestinal disorders Does not appear to increase the incidence of opportunistic infections or malignancies CYTOKINE INHIBITORS TNF inhibitors (disease modifiers to treat rheumatoid arthritis) Etanercept Recombinant version of TNF receptor Infliximab Chimeric human/murine anti-TNF monoclonal antibody Anakinra Human IL-1 receptor antagonist Disease modifier agent for Rheumatoid arthritis MUROMONAB-CD3 Description Murine monoclonal antibody against CD3 component of Tcell–receptor signal-transduction complex Mechanism Monoclonal IgG2a antibody Binds to the T cell receptor-CD3-complex on the surface of circulating T cells, interfering with the receptor-antigeninteraction Resulting in a transient activation of T cells, release of cytokines, and blocking of T-cell proliferation and differentiation T-cell function usually returns to normal within approximately 48 hours of discontinuation of therapy. MUROMONAB-CD3 PRESCRIBED FOR Renal, heart and liver transplant SIDE EFFECT Severe cytokine-release syndrome, pulmonary edema, acute renal failure, gastrointestinal disturbances, changes in central nervous system Pyrexia(fever),myalgia, nausea and diarrhoea ALEMTUZUMAB DESCRIPTION Humanized monoclonal antibody against CD52, a 25-to-29-kD membrane protein MECHANISM Binds to CD52 on all B and T cells, most monocytes, macrophages, and natural killer cells, causing cell lysis and prolonged depletion ALEMTUZUMAB PRESCRIBED FOR CLL and T cell lymphoma SIDE EFFECT Hypotension, fever, shortness of breath, bronchospasm, chills, and/or rash POLYCLONAL ANTIBODY Antithymocyte Antithymocyte Thymoglobulin®) Description globulin-equine globulin-rabbit (Atgam®), (RATG, Polyclonal antibodies are directed against lymphocyte antigens, directed against multiple epitopes POLYCLONAL ANTIBODY MECHANISM Agents contain antibodies specific for many common T cell antigens including CD2, CD3, CD4, CD8, CD11a, CD18 Effectively depletes T-cell concentration in the body through complement-dependent cytolysis and cell mediated opsonization Following with T cell clearance from the circulation by the reticuloendothelial system POLYCLONAL ANTIBODY SIDE EFFECT Leukopenia serum sickness (cross-reactivity with other tissue antigens) adversely affects the ability of the patient to make antibodies against foreign protein thrombocytopenia pruritis fever arthralgias opportunistic infections malignancies ANTI –D IMMUNEGLOBULIN Human Ig G having high titre of antibodies against Rh (D) antigen It binds the Rho Ag (-) antibody formation in Rh -ve individuals It is used for prevention of postpartum /post –abortion formation of antibodies in Rho-D negative. Administered within 72 hrs of delivery /abortion, such treatment prevents Rh hemolytic disease in future offspring. It has also been given at 28th week of pregnancy Never be given to Rh +ve individuals THANK YOU -PHARMA WORLD