* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Elevated Hepatic Insulin Extraction in Essential Hypertension
Metabolic syndrome wikipedia , lookup
Hypoglycemia wikipedia , lookup
Diabetes management wikipedia , lookup
Insulin resistance wikipedia , lookup
Gestational diabetes wikipedia , lookup
Diabetic ketoacidosis wikipedia , lookup
Complications of diabetes mellitus wikipedia , lookup
646
Elevated Hepatic Insulin Extraction in
Essential Hypertension
Alexandra Kautzky-Willer, Giovanni Pacini, Michael Weissel, Maria Capek,
Bernhard Ludvik, and Rudolf Prager
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
Insulin resistance, hyperinsulinemia, and dyslipidemia are common characteristics of patients with
untreated hypertension. However, the link between the vascular and metabolic disturbances is still
unclear. To provide further insights into the metabolic picture of subjects with hypertension, we evaluated
insulin resistance, pancreatic secretion, and hepatic extraction of the hormone in 16 untreated patients
with essential hypertension before and after 12-16 weeks of drug treatment in comparison with 16 age-,
sex-, and body weight-matched normotensive control subjects. All subjects underwent an oral and a
frequently sampled intravenous glucose tolerance test. Metabolic parameters were calculated by the
minimal model technique. The hypertensive patients exhibited a highly reduced tissue insulin sensitivity
(2.6±0.4 versus 9.6±1.9 104 min~V[microunits/mL];p<0.001). The basal secretion rate (70+11 versus
35±5 pmol/L per minute) and the total amount of prehepatically secreted insulin (32±4 versus 16±2
nmol/L in 4 hours) were significantly increased in the hypertensive patients compared with the control
subjects (p<0.01), whereas the posthepatic insulin delivery rate was not significantly different between
the two groups (4.9±0.6 versus 3.5 ±0.3 nmol/L in 4 hours). Hepatic insulin extraction was found to be
significantly elevated in the hypertensive patients compared with control subjects (81±4% versus 69±3%,
p<0.04). Increased hepatic insulin extraction partially ameliorated B cell hypersecretion in hypertensive
patients. After 12-16 weeks of drug treatment, the blood pressure was normalized, but the metabolic
profile of the patients remained unchanged. We conclude that elevated insulin extraction in the liver is a
specific characteristic of individuals with essential hypertension and partially compensates pancreatic B
cell hypersecretion. {Hypertension 1993;21:646-653)
KEY WORDS • hypertension, essential • insulin sensitivity • hepatic insulin extraction
T
he striking clinical association among hypertension, hyperinsulinemia, and impaired glucose
tolerance has attracted considerable attention
in recent years,1"3 but despite great scientific effort the
etiology and pathogenesis of these diseases and the link
between high blood pressure and the metabolic aberrations are still unknown.4-8 Because these conditions
represent predictors for cardiovascular disease and
early mortality, it would be of great interest to elucidate
the hyperinsulinemia-hypertension link. Moreover, it is
still controversial whether drug treatment leads to
changes in the metabolic status of patients with essential hypertension and has any effect on coronary artery
disease at all.
Although several studies give evidence of high peripheral insulin levels in hypertension,5-6-8 there are
only a few investigations on dynamic beta cell function
and insulin resistance in this state. 6 To our knowledge,
the role of hepatic insulin extraction in the pathogen-
From the Second Medical Department, University of Vienna
(Austria), and the Institute of Systems Theory and Biomedical
Engineering (LADSEB-CNR), Padua, Italy.
Supported by the Osterreichische Nationalbank Jubilaumsfond
4249.
Address for correspondence: A. Kautzky-Willer, MD, Department of Medicine III, University of Vienna, Wahringer Giirtel
18-20, 1090 Vienna, Austria.
Received August 19, 1992; accepted in revised form December
28, 1992.
esis of hyperinsulinemia in essential hypertension has
not been elucidated so far. Therefore, this study
focused on beta cell insulin secretion, the role of
hormone extraction in the liver, and insulin sensitivity
in patients with essential hypertension before and
12-16 weeks after treatment with calcium channel
blockers, angiotensin converting enzyme inhibitors, or
both, drugs that are proposed to lack significant
metabolic side effects.
Only invasive methods exist for obtaining direct
information on prehepatic insulin release and insulin
extraction by the liver, such as catheterization of the
portal vein for sampling in situ. The most widely used
noninvasive approach has been the C peptide-toinsulin molar ratio; however, the limitations of its use
as an indicator of hepatic extraction are extensively
discussed by Polonsky and Rubenstein.9 We exploited
a noninvasive method based on two minimal mathematical models: one of C peptide secretion and kinetics10 and one of insulin delivery and kinetics.11 This
technique, which includes only a few assumptions
based on accepted physiological concepts, allows the
interpretation of plasma C peptide and peripheral
insulin concentration data during a frequently sampled intravenous glucose tolerance (FSIGT) test in
humans and provides an estimation of the time courses
of the prehepatic insulin secretion rate and the hormone degradation in the liver.
Kautzky-Willer et al
TABLE 1. Clinical Characteristics of Hypertensive Patients and
Nonnotensive Control Subjects
Variable
n (men/women)
Age (years)
BMI (kg/m2)
SBP (mm Hg)
DBP (mm Hg)
Triglycerides (mmol/L)
Cholesterol (mmol/L)
HDL cholesterol (mmol/L)
LDL cholesterol (mmol/L)
Uric acid (jimol/L)
Hb A, c (%)
Hypertensive
Normotensive
16 (9/7)
46±4
26±1
16 (10/6)
167±8
102±5
1.8±0.2
6.5+0.4
1.27±0.1
3.8±0.3
330±18
5.4±0.2
41 ±3
24±1
125 ±2
79±2
1.1+0.1
4.7±0.4
1.25±0.1
3.1 ±0.2
270+12
4.6±0.2
P
NS
NS
<0.0001
< 0.0005
<0.04
<0.01
NS
<0.05
<0.04
<0.01
BMI, body mass index; SBP, systolic blood pressure; DBP,
diastolic blood pressure; HDL, high density lipoprotein; LDL, low
density lipoprotein. Values are mean±SEM.
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
Methods
Subjects
We studied 16 nondiabetic patients (Table 1) with
essential hypertension (nine men, seven women; age
range, 32-65 years; body mass index [BMI], 26.0± 1 kg/m2;
mean systolic blood pressure [SBP], 167±8 mm Hg; mean
diastolic blood pressure [DBP], 102±5 mm Hg). Essential
hypertension was diagnosed when SBP exceeded 160
mm Hg and DBP exceeded 95 mm Hg on at least three
measurements by the same physician on separate days.
The blood pressure values are mean values of 24-hour
continuous blood pressure monitoring (Reynolds Accutracker II, Medical LTD, Hertford, UK). A complete
medical workup was carried out to exclude secondary
forms of hypertension. Renal, liver, and endocrine functions were normal. The hypertensive patients were recruited from the outpatient clinic; none had any disease
other than hypertension nor any family history of diabetes.
The control population was recruited from within the
paramedical staff and consisted of 16 healthy subjects
matched for sex, age, and body weight (10 men, six
women; age range, 27-62 years; BMI, 24±1 kg/m2; SBP,
125±2 mm Hg; DBP, 79±2 mm Hg; see Table 1).
After 12-16 weeks of treatment with calcium channel
blockers (nifedipine), angiotensin converting enzyme
inhibitors (enalapril), or both, the hypertensive patients
reached normal blood pressure levels (SBP, 142±6
mm Hg; DBP, 88±3 mm Hg;p<0.01) and were restudied to assess whether changes in their metabolic status
occurred. The normalization of blood pressure levels
was confirmed by repetition of 24-hour blood pressure
monitoring, as well as by training electrocardiogram.
Oral Glucose Tolerance Test
All subjects underwent a standard (75 g) oral glucose
tolerance test after an overnight fast. Blood was drawn
at 0 minutes (before the glucose was drunk) and 30, 60,
90, 120, and 180 minutes after the glucose load for
measurement of glucose and insulin levels.
Frequently Sampled Intravenous Glucose
Tolerance Test
For each subject, tests were started at 8 AM after an
overnight fast. A catheter was inserted into an antecu-
Insulin Metabolism in Essential Hypertension
647
bital vein for blood sampling and into a contralateral
antecubital vein for glucose injection. Basal samples
were drawn at —20, —10, and —1 minutes. At time 0,
glucose (300 mg/kg) was injected in 1 minute. Additional samples were collected at 2, 3,4,5,6,8,10,12,14,
16,19,22, 25,30,40,50,60, 70,80,90,100,110,120,140,
160, 180, 210, and 240 minutes. Blood was rapidly
centrifuged and glucose immediately measured by the
glucose oxidase method with an automated glucose
analyzer (Beckman Instruments Inc., Fullerton, Calif.).
The remaining plasma was stored at -20°C for later
insulin and C peptide determinations. Insulin (Pharmacia LKB Biotechnology, Uppsala, Sweden) and C peptide (Byk Sangtec, Dietzenbach, FRG) were measured
by commercially available radioimmunoassays.
Data Analysis
The FSIGT test data were submitted to computer
programs that calculate the characteristic metabolic
parameters by fitting glucose, insulin, and C peptide
data to the minimal models that describe the time
courses of glucose and insulin12 and of C peptide10
concentrations. The models assume a first-order linear
kinetics for both insulin and C peptide and a glucosecontrolled biphasic release from the B cell. The two
models have previously been described in detail10-15;
here, we briefly present what is useful to the understanding of their application in this study.
Glucose disappearance minimal model. The glucose
disappearance minimal model12 accounts for the effect
of insulin and glucose on glucose disappearance after
the exogenous glucose injection. It provides two parameters: Sr is the insulin sensitivity index (per minute per
microunit per milliliter), defined as the ability of insulin
to enhance glucose disappearance and to inhibit hepatic
glucose production; SG is glucose effectiveness (per
minute), defined as the ability of glucose per se to
enhance its own disappearance and to inhibit glucose
production at basal insulin.
C peptide minimal model. The C peptide minimal
model10 accounts for the effect of glucose on C peptide
concentration during an FSIGT test by describing the
ability of the beta cells to secrete C peptide in response
to the glycemic stimulus and C peptide kinetics after its
entry into the peripheral circulation. The model provides the time course of C peptide secretion, CPS(t)
(picomole per liter per minute), and the value of
parameters * , (picomole per liter per minute per
milligram per deciliter) and <&2 (picomole per
liter • minute' 2 per milligram per deciliter), which describe the dynamic (suprabasal) first- and second-phase
beta cell (prehepatic) sensitivity to glucose, respectively.
The former represents the amount of C peptide per unit
volume immediately released per unit change in plasma
glucose, and the latter is the sensitivity to glucose of the
second-phase pancreatic secretion. In addition, the
model provides parameter koi, which is the C peptide
fractional clearance rate (per minute), i.e., the peptide
disappearance in unit time per unit volume.
Insulin secretion and hepatic extraction. Because insulin and C peptide are secreted in equimolar fashion, the
time course of beta cell insulin secretion equals that of
C peptide, CPS(t), and basal insulin secretion rate, BSR
(picomole per liter per minute), may be computed by
multiplying the clearance rate ko, by the basal C peptide
648
Hypertension
Vol 21, No 5 May 1993
TABLE 2. Comparison of Oral Glucose Tolerance Test Results for Normotensive Control Subjects and
Hypertensive Patients Before and After Drug Treatment
Hypertensive
Variable
Glucose
Basal (mmol/L)
At 120 minutes (mmol/L)
Stimulated! (mol/L in 3 hours)
Insulin
Basal (pmol/L)
At 120 minutes (pmol/L)
Stimulated! (nmol/L in 3 hours)
Normotensive
Before treatment
After treatment
P*
4.7±0.2
5.5 ±0.3
2.1+0.3
5.5+0.08
7.7+0.6
5.3 ±0.5
5.4 ±0.08
7.3±0.5
4.6+0.4
<0.05
<0.001
< 0.0005
60±12
162+18
24±3
102+12
654+114
90+12
120±18
600 ±84
78±6
<0.02
<0.001
< 0.0001
Values are mean±SEM. n = \6 for both hypertensive patients and normotensive control subjects.
'Difference between hypertensive patients before treatment and normotensive control subjects.
fCalculated as suprabasal area under the concentration curve.
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
concentration. BSR, <J>,, and 4>2 give a quantitative
description of the individual components (basal and
dynamic) of the beta cell secretion.
A previously introduced minimal model to analyze
FSIGT insulin data11 provides the time course of posthepatic insulin delivery, IDR(t). Therefore, the time
course of the percent hepatic insulin extraction, HE(t),
may be computed as the difference between CPS(t) and
IDR(t), normalized to CPS(t).10
Calculations
The estimation of parameters was carried out with a
nonlinear least-squares estimation technique by the
computer program MINMOD,14 which was modified to
account also for C peptide data.15 A constant variance
structure was assumed for the measurement error: the
coefficient of variation assessed in our lab of a single
determination was ±1.5% for glucose, ±7% for insulin,
and ± 12% for C peptide. Accuracy and precision of the
estimates were evaluated according to the validation
criteria of model identification.16
Parameters and time courses were given by the model
analysis per unit volume, rendering correct the comparison between groups with different distribution volumes.
The method is also independent of the glucose dose,
providing enough glucose was administered to adequately stimulate beta cell insulin release. In our experience, glucose doses greater than 20 g yield sufficiently
high dynamic insulin profiles even in low responding
subjects.
The initial distribution volume for glucose (VD, liters)
was calculated as the ratio between the injected dose of
glucose and the model extrapolation of glycemia to time
zero. Parameter SG, the glucose effectiveness, can be
split into two components (both per minute): 1) basal
insulin effect (BIE) on glucose disappearance and calculated as basal insulin times Si and 2) the glucose
effectiveness at zero insulin (GEZI), i.e., the contribution to glucose disappearance of non-insulin-dependent
glucose uptake, calculated as SG-BIE.17
The total amounts of insulin secreted by the beta cell
(TIS, nanomole per liter in 4 hours) and of insulin
delivered into the periphery (TID, nanomole per liter in
4 hours) were computed by the integral, between 0 and
240 minutes, of CPS(t) and IDR(t), respectively.
Differences in mean values between the hypertensive
patients and normotensive control subjects were tested
for significance by analysis of variance. Statistical comparison of hypertensive patients before and after medication was calculated by paired Student's t test. Correlation coefficients were calculated by linear regression
analysis. NS means a value of /?>0.05. All data and
results are given as mean±SEM.
Results
Metabolic Profile
Compared with control subjects, the hypertensive
patients featured significantly higher triglyceride levels
(/7<0.03) and significantly higher total (/><0.01) and
low density lipoprotein (LDL) (p<0.05) cholesterol
levels, whereas the high density lipoprotein (HDL)
cholesterol levels did not differ between the two groups
(Table 1). Hypertensive patients showed moderate microalbuminuria (98±8 mg/L in 24-hour sampled urine).
The levels of uric acid were in the normal range in
hypertensive patients (Table 1) but were significantly
elevated compared with control subjects (/?<0.04).
Body fat distribution in the hypertensive patients evaluated by measurement of the waist-to-hip-girth ratio
ranged from 0.78 to 0.95. The oral glucose tolerance test
indicated that no hypertensive patient had overt diabetes (Table 2). According to National Diabetes Data
Group criteria,18 seven hypertensive patients exhibited
impaired glucose tolerance. The remaining nine patients showed normal glucose tolerance. Comparing the
two subgroups with normal and impaired glucose tolerance, we found no significant differences in blood
pressure, cholesterol, triglycerides, uric acid levels, insulin secretion, and insulin resistance. Neither BMI nor
the waist-to-hip-girth ratio correlated with blood pressure, cholesterol levels, or triglyceride levels in hypertensive patients.
After 12-16 weeks of drug treatment with angiotensin
converting enzyme inhibitors, calcium channel blockers,
or both, there was no significant change in the basal or
stimulated insulin and glucose concentration curves (Table 3) as well as in the lipid concentrations (triglycerides,
1.8±0.2 versus 2.2±0.3 mmol/L; total cholesterol, 6.5±0.4
versus 6.05±0.3 mmol/L; HDL cholesterol, 1.17±0.1 versus 1.4±0.1 mmol/L; LDL cholesterol, 3.8±0.3 versus
4.2 ±0.4 mmol/L; hypertensive patients before versus after
Kautzky-Willer et al Insulin Metabolism in Essential Hypertension
649
TABLE 3. Model Estimated and Calculated Parameters from Frequently Sampled Intravenous Glucose Tolerance
Test for Normotensive Control Subjects and Hypertensive Patients Before and After Treatment
Hypertensive
Model parameter
Glucose
S[ (min"V[microunit/mL])
SG (l(r 3 mirr1)
V D (% body wt)
Insulin secretion
BSR (pmol/L per minute)
<t>, (pmol/L min~'/[mg/dL])
<D2 (lO" 3 pmol/L min- 2 /[mg/dL])
TIS (nmol/L in 4 hours)
Hepatic extraction
Basal (% secreted insulin)
HIE (% secreted insulin)t
Normotensive
Before treatment
After treatment
P*
9.6±1.9
32±3
17.5±0.6
2.6±0.4
3.6±0.5
18±2
17.2±0.5
<0.001
<0.001
71±11
189±32
55 + 11
30±3
<0.01
NS
NS
< 0.005
84±2
80±4
<0.04
<0.04
35 ±5
175±24
15±2
17.2+0.5
70±n
48±9
16±2
186±30
47±5
32±4
71±3
69±3
83±3
81+4
NS
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
Si, insulin sensitivity; SG, glucose effectiveness; VD, volume of glucose distribution; BSR, basal insulin secretion rate;
<t>b first-phase B cell sensitivity to glucose; <J>2, second-phase B cell sensitivity to glucose; TIS, total insulin secretion;
HIE, hepatic insulin extraction. Values are mean±SEM. n = 16 for hypertensive patients and normotensive control
subjects.
'Difference between hypertensive patients before treatment and normotensive control subjects.
tAverage extraction in 240 minutes.
treatment). Eight of the 16 patients received angiotensin
converting enzyme inhibitors, five patients were treated
with calcium channel blockers, and three hypertensive
patients were on a combination of both antihypertensive
drugs. In each subgroup of hypertensive patients, glucose,
insulin levels, insulin secretion, and lipid parameters also
remained unchanged.
Hb A k levels in hypertensive patients were significantly
elevated compared with control subjects (5.4±0.2% versus
4.6±0.1%,/?<0.01, hypertensive versus control), reflecting
the higher blood glucose levels in this patient group. The
Hb Alc levels showed a significant correlation with triglyceride (/><0.03, r=0.6) levels and correlated inversely with
HDL cholesterol (/?<0.01, r=-0.7).
Neither sex nor the degree of glucose tolerance nor
body weight affected blood pressure levels.
Frequently Sampled Intravenous Glucose Tolerance
Test and Model Parameters
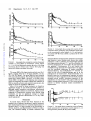
During the FSIGT test, we observed significantly
higher C peptide, insulin, and glucose levels in the
hypertensive patients compared with normotensive control subjects (Figure 1). Basal levels were (hypertensive
versus control) C peptide, 1.1 ±0.1 versus 0.55 ±0.06
nmol/L (/?<0.001); insulin, 70±9 versus 42±5 pmol/L
(/?<0.05); and glucose, 5.3±0.2 versus 4.8±0.1 mmol/L
(p<0.05). Integrated insulin concentration was 6.3±0.9
and 3.5±0.6 nmol/L in 240 minutes, p<0.05, in hypertensive patients and controls, respectively.
Concentration curves of hypertensive patients with
normal and impaired glucose tolerance were not significantly different.
Insulin sensitivity and glucose effectiveness. The hypertensive group featured a highly significantly reduced
insulin sensitivity (Table 2), which correlated inversely
with the Hb Alc levels (r=-0.65,/?<0.02). The glucose
effectiveness was halved in hypertensive patients and
showed a significant relation with HDL cholesterol
(r=0.65, /?<0.02). Both components of glucose effec-
tiveness, BIE (0.0025±0.0003 versus 0.0064±0.0013
min M , /?<0.001) and GEZI (0.0126±0.0025 versus
0.0251 ±0.0033 min"', p<0.001), were significantly
lower in the hypertensive patients compared with normotensive control subjects.
Initial glucose distribution volume was 13.2 ±1 L in
hypertensive patients and 12.1 ±0.6 L in normotensive
control subjects (NS), corresponding to 17.2±0.5% and
17.5±0.6% of body weight, respectively.
Insulin secretion. No differences were found in the C
peptide fractional clearance rate between the control and
hypertensive groups (0.067±0.007 versus 0.060±0.007
min~', respectively). The calculated time courses of prehepatic insulin secretion are shown in the top panel of
Figure 2. The basal C peptide secretion rate, which equals
basal insulin prehepatic release, was significantly higher in
hypertensive patients than in normotensive control subjects (Table 3). No significant differences were found in
the dynamic sensitivities to glucose of the first- and
second-phase insulin secretion. The total amount of released C peptide, which equals that of insulin, was twice as
much in the hypertensive group as in the control group.
Insulin secretion correlated with Hb A,c (r=0.82,p<0.01).
Hepatic extraction. The time course of estimated
hepatic insulin extraction is shown in Figure 3. Basal
hepatic extraction was significantly elevated in hypertensive patients as well as its mean value (HIE) during
the entire test (Table 3). HIE correlated with both basal
peptide secretion rate (r=0.55,/><0.05) and total insulin
secretion (r=0.6, /><0.05). The rate of posthepatic delivery of insulin is shown in the bottom panel of Figure
2. The area under this curve (total insulin delivery)
represents the amount of insulin in the periphery. Total
insulin delivery, although elevated in hypertensive patients, was not statistically significant compared with
control subjects (4.9±0.6 versus 3.5±0.3 nmol/L in 4
hours, respectively).
Comparing hypertensive patients with normal and
impaired glucose tolerance, we could not find any
significant difference in the model parameters.
650
Hypertension
Vol 21, No 5 May 1993
a
180
240
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
60
120
180
240
60
120
180
240
FIGURE 2. Line graphs show average time courses of beta
cell insulin release (prehepatic) and posthepatic insulin delivery for normotensive control subjects (O—O) and hypertensive patients (•—•) (mean±SEM) as predicted by the minimal model.
FIGURE 1. Line graphs show average time courses of measured
variables as mean±SEM of normotensive control subjects
(O—O; n=16) and hypertensive patients (•—•; n=16) during
intravenous glucose tolerance tests. Glucose (0.33 g/kg) was
started at 0 minutes and lasted 30 seconds.
The mean BMI of the hypertensive patients was 26 ± 1
kg/m2, ranging from 19 to 32 kg/m2 (median, 25; Ql,
22.5; Q3, 30.5 kg/m2). The mean BMI did not correlate
with any model parameter. We divided the hypertensive
group into a leaner (BMI <24 kg/m2, n=8) and a more
obese (BMI >24 kg/m2, n=8) subgroup and compared
these two subgroups to further evaluate the influence of
BMI on insulin secretion and sensitivity parameters, but
again we found no significant difference.
After 12-16 weeks of drug treatment, we found no
significant change in any model parameter (Table 3),
although insulin sensitivity and glucose effectiveness
exhibited a slight tendency to ameliorate. Again, in each
subgroup of hypertensive patients with specific drug
treatment, we saw no significant change in insulin
sensitivity and glucose effectiveness or in any other
model parameter.
Discussion
In recent years, interest has been focused on the
possible role of glucose intolerance, insulin resistance,
and insulin hypersecretion in essential hypertension.1'5
Because there is a large overlap in the prevalence of
obesity, diabetes, and hypertension in Western civilization, the common finding of insulin resistance and
hyperinsulinemia in hypertensive patients is not surprising. However, recent studies have shown that insulin
resistance is also a problem of normal-weight nondiabetic hypertensive patients,219"21 and the mortality risk
has been proposed to be even higher in lean hypertensive patients.22 Furthermore, it is well known that
established drug therapies for hypertension can aggravate the metabolic syndromes of insulin resistance,
hyperinsulinemia, and dyslipidemia.23-26 Interest focused on the role of hyperinsulinemia per se in the
pathogenesis of hypertension. Hyperinsulinemia as a
primary cause for or compensatory response to insulin
resistance has been suggested to elevate blood pressure
through several actions, including activation of the
sympathetic nervous system27 and a direct effect on the
kidney, causing sodium retention.28 Thus it was of great
interest to further elucidate insulin metabolism in
hypertension.
u
60
120
180
240
FIGURE 3. Line graph shows average time course of hepatic
insulin extraction for normotensive control subjects (o—O)
and hypertensive patients (•—•) (mean±SEM).
Kautzky-Willer et al Insulin Metabolism in Essential Hypertension
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
Impaired glucose metabolism in the hypertensive
patients was documented by significantly higher glucose
levels during the glucose tolerance tests and by significantly elevated glycosylated hemoglobin, although the
Hb Alc levels of the hypertensive patients were still in
the normal range. The degree of hyperglycemia, measured by Hb A lc , was significantly correlated with insulin secretion and insulin resistance, as well as the LDL
cholesterol and triglyceride levels. In this context, it is of
considerable interest that the prevalence of hypertension was found to be clearly increased with the Hb Alc
levels among older women in the original cohort of the
Framingham Heart Study.29
From the analysis of FSIGT data, the hypertensive
patients were severely insulin insensitive, featuring insulin
sensitivity values reduced by 75% compared with control
subjects. The other parameter strictly related to glucose
disappearance, i.e., glucose effectiveness, was reduced by
51% in the hypertensive patients. The importance of this
parameter in overall glucose metabolism has been recently
explored.17 Because glucose effectiveness measures the
ability of glucose per se to increase glucose utilization30
and to decrease endogenous glucose production,31 in the
absence of changes in plasma insulin, abnormalities in the
processes at the level of peripheral tissues as well as in the
liver may be accounted for by the impaired glucose
effectiveness. A study in experimental animals showed
that the relative contribution of glucose per se is at least as
important as the insulin-dependent glucose uptake in
normalizing the glycemia after an intravenous glucose
load.32 In addition, the synergistic, independent, and almost comparable effects of insulin sensitivity and glucose
effectiveness on glucose disappearance were also demonstrated in obese33 and cirrhotic34 humans. It is interesting
to notice that the components of insulin sensitivity, i.e.,
the BIE and the GEZI, were both reduced in hypertensive
patients by a similar proportion. The percent ratio of
GEZI to insulin sensitivity represents that part of tissue
glucose uptake occurring independent of insulin. This
ratio was 78% in control subjects and 84% in hypertensive
patients, very similar to the 77% found by Kahn and
coworkers17 in normotensive men using a similar approach
(glucose plus tolbutamide intravenous glucose tolerance
test) and to the 83% found by Baron and coworkers35 in
men using a glucose clamping technique. Our results show
that the efficiency of glucose uptake by the whole body is
reduced in the full range of insulin levels, from "zero"
insulin to hyperinsulinemia, leading to the conclusion that
hypertensive patients exhibit a marked tissue glucose
resistance.
We must emphasize that the findings of the present
study are based on the analysis of "regular" FSIGT
data, i.e., without tolbutamide, the use of which has
been suggested by Bergman and coworkers.13 The use of
pharmacological agents that increase the B cell response is especially recommended when dynamic insulin profile is very low. In such a situation, in fact, insulin
sensitivity and glucose effectiveness are likely to be
estimated with no accuracy. This is not the case for our
data; no subject exhibited low insulin profiles. In addition, we were interested in the B cell and liver behavior
toward endogenous insulin, which is the novelty of the
present study, and tolbutamide, acting on the B cell,
would have disturbed the main purpose of this work.
651
Decreased insulin sensitivity and glucose effectiveness resulted in postload hyperglycemia, which produces a larger-than-normal stimulation of the B cells,
leading to insulin hypersecretion. Hyperinsulinemia was
markedly evident in the hypertensive patients during
both the oral and intravenous glucose tolerance tests;
for instance, the total area under the FSIGT insulin
concentration curve was twice as great as that of
normotensive control subjects.
The insulin hypersecretion in the patients with high
blood pressure could be factored out in its components
by our modeling method. During the dynamic hyperglycemic phases, the B cell of the hypertensive patients
reacted as that of control subjects; in fact, both of the
components <!>, and <J>2 were not different in the two
groups. Thus, despite hypertension, the amount of
first-phase releasable insulin was maintained intact.
Similar values of parameter <t>2 also lead to the conclusion that the capacity to synthesize newly releasable
hormone was not impaired. What seems to be unequivocal is the elevated basal secretory rate. The increased
basal glucose (on average, 0.8 mmol/L) overstimulated
the B cell in steady-state conditions (basal secretory
rate of hypertensive patients was twice that of control
subjects), with a sustained effect probably due to the
overt insulin resistance demonstrated in the hypertensive patients.
The clearance of C peptide was not different in the
two groups, showing that the kinetic behavior of C
peptide was not affected by essential hypertension. The
clearance of C peptide of the normotensive control
subjects also was similar to that found by analyzing the
disappearance curve of bolus-injected exogenous biosynthetic human C peptide in normotensive volunteers.36 This fact made us confident that our findings
about insulin through C peptide analysis are not affected by bias due to a different kinetic behavior of C
peptide in the two groups.
Although insulin secretion was more than doubled in
the hypertensive patients, the posthepatic basal insulin
delivery rate did not exhibit a marked difference between the two groups, and the posthepatic total insulin
delivery was only insignificantly higher in the hypertensive patients compared with control subjects. This fact
brings the focus on the role the liver plays in modulating
insulin appearance in the periphery. Hepatic degradation of the hormone was elevated by 15% in hypertensive patients compared with control subjects and significantly correlated with insulin secretion. Higher insulin
extraction obviously lowered peripheral hyperinsulinemia in the hypertensive patients. Therefore, the elevated insulin extraction by the liver can be seen as a
compensating mechanism to avoid excessive peripheral
hyperinsulinemia. As a matter of fact, the compensatory
effect between B cell insulin secretion and liver degradation has been suggested in a study on elderly people,
in which a reduced secretion and a decreased extraction
were considered responsible for maintenance of normal
peripheral insulinemia.37
Interestingly, the body weight, measured by BMI, was
not correlated with glucose tolerance, insulin sensitivity,
insulin secretion, and the lipid status in the hypertensive
patients investigated in this study. In fact, other investigators also reported severe insulin resistance in lean
hypertensive patients,6 and a strong association between
652
Hypertension
Vol 21, No 5 May 1993
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
elevated blood pressure and insulin insensitivity irrespective of the body weight was also documented in
nonobese non-insulin-dependent diabetes mellitus patients with hypertension.38 Thus, insulin resistance and
associated insulin hypersecretion as well as dyslipidemia
seem to be characteristic features of essential hypertension irrespective of the the degree of obesity.
Angiotensin converting enzyme inhibitors, calcium
channel blockers, or both were used for drug therapy,
because they should not have adverse effects on glucose
metabolism.39-41 Calcium entry blockers are neutral or
slightly positive on glucose insulin,42 and angiotensin
converting enzyme inhibitors were shown to have no
effect43 or to improve insulin sensitivity measured by a
clamp technique.39 Medical treatment and normalization of blood pressure in all hypertensive patients had
no significant effect on any of the measured metabolic
parameters, and no substantial change in the comprehensive metabolic portrait of the hypertensive patients
was observed.
Subtle changes in insulin sensitivity, insulin secretion,
and lipid parameters induced by specific drug treatment
as seen by others39'42 might have been missed in this
study because of the small sample size of patients
treated with angiotensin converting enzyme inhibitors,
calcium channel blockers, or both. On the other hand, a
beneficial effect of angiotensin converting enzyme inhibitors on insulin secretion and insulin sensitivity is not
consistently reported.26-43 Recent studies using the
clamp technique showed that treatment with angiotensin converting enzyme inhibitors failed to change insulin
sensitivity in hypertensive patients with non-insulindependent (type II) diabetes mellitus41 as well as in
nondiabetic patients with essential hypertension44 despite effective reduction of blood pressure levels. In
these studies, however, the patients were restudied 8
weeks41 or 12 weeks44 after antihypertensive drug treatment was begun. Thus, the lack of metabolic improvement after pharmacological treatment reported in these
studies as well as in this one (12-16 weeks) might be due
to the short duration of drug therapy.
It is still controversial whether a direct and independent relation between blood pressure and insulin hypersecretion and/or insulin resistance really exists. A causeand-effect relation between hyperinsulinemia and
hypertension has not been proved, and several studies
suggest that only a weak correlation exists between
plasma insulin concentration and blood pressure in
normal humans and that blood pressure does not appear to be very sensitive to changes in blood insulin.45-49
Maybe other links between the vascular and metabolic
abnormalities exist, which are responsible for the clustering of insulin hypersecretion and hypertension; e.g.,
hemodynamic alterations and vascular rarefaction in
essential hypertension might be a possible cause of
insulin resistance.50
In conclusion, patients with hypertension are characterized by insulin resistance, insulin hypersecretion, and
dyslipidemia irrespective of their body weight. Medical
treatment of hypertension at least with angiotensin
converting enzyme inhibitors and calcium channel
blockers has no effect on these metabolic abnormalities.
Hepatic insulin extraction in hypertension is elevated
and may serve as a compensatory mechanism to avoid
excessive peripheral hyperinsulinemia in these patients.
References
1. Reaven GM: Role of insulin resistance in human disease. Diabetes
Care 1991;14:195-202
2. Zavaroni I, Bonora E, Pagliara M, DalFAglio E, Luchetti L, Buonanno G, Bonati PA, Bergonzani M, Gnudi L, Passeri M, Reaven
G: Risk factors for coronary artery disease in healthy persons with
hyperinsulinemia and normal glucose tolerance. N Engl J Med
1989;320:702-705
3. Fontbonne AM, Eschwege EM: Insulin and cardiovascular disease:
Paris Prospective Study. Diabetes Care 1991;14:461-469
4. Drury PL: Diabetes and arterial hypertension. Diabetologia 1983;
24:1-9
5. Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, Shitrit
A, Fuchs Z: Hyperinsulinemia: A link between hypertension, obesity, and glucose intolerance. J Clin Invest 1985;75:809-817
6. Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini
M, Graziadei L, Pedrinelli R, Brandi L, Berilaqua S: Insulin resistance in essential hypertension. N Engl J Med 1987;7:317-350
7. O'Hare JA: The enigma of insulin resistance and hypertension. Am
3 Med 1988;84:505-510
8. Hall JE, Coleman TG, Mizelle HL: Does chronic hyperinsulinemia
cause hypertension? Am Hypertens 1989;2:171-173
9. Polonsky RS, Rubenstein AH: C-peptide as a measure of the
secretion and hepatic extraction of insulin: Pitfalls and limitations.
Diabetes 1984;33:486-494
10. Cobelli C, Pacini G: Insulin secretion and hepatic extraction in
humans by minimal modeling of C-peptide and insulin kinetics.
Diabetes 1988;37:223-231
11. Toffolo G, Bergman RN, Finegood DT, Bowden CR, Cobelli C:
Quantitative estimation of beta cell sensitivity to glucose in the
intact organism: A minimal model of insulin kinetics in the dog.
Diabetes 1980;29:979-990
12. Bergman RN, Phillips LS, Cobelli C: Physiologic evaluation of
factors controlling glucose tolerance in man. J Clin Invest 1981 ;68:
1456-1467
13. Bergman RN, Prager R, Volund A, Olefsky J: Equivalence of the
insulin sensitivity index in man derived by the minimal model
method and the euglycemic glucose clamp. J Clin Invest 1987;79:
790-800
14. Pacini G, Bergman RN: MINMOD: A computer program to calculate insulin sensitivity and pancreatic responsitivity from the
frequently sampled intraveneous glucose tolerance test. Comput
Methods Programs Biomed 1986;23:113-122
15. Pacini G, Cobelli C: Estimation of beta cell secretion and insulin
hepatic extraction by the minimal modelling technique. Comput
Methods Programs Biomed 1990;32:241-248
16. Carson ER, Cobelli C, Finkelstein L: The Mathematical Modelling
of Metabolic and Endocrine Systems. New York, John Wiley & Sons,
Inc, 1983
17. Kahn SE, KloffLJ, Schwartz MW, Beard JC, Bergman RN, Taborsky GJ, Porte D: Treatment with a somatostatin analog decreases
pancreatic beta cell and whole body sensitivity to glucose. J Clin
Endocrinol Metab 1990;71:994-1002
18. National Diabetes Data Group: Classification and diagnosis of
diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-1057
19. Ferrannini E, DeFronzo RA: The association of hypertension,
diabetes and obesity: A review. J Nephrol 1989;1:3-15
20. Bonora E, Zavaroni I, Alpi O, Pezzarossa A, Bruschi F, Dall'Aglio
E, Guerra L, Coscelli C, Butturini U: Relationship between blood
pressure and plasma insulin in non-obese and obese non-diabetic
subjects. Diabetologia 1987;30:719-723
21. Pollare T, Lithell H, Berne C: Insulin resistance is a characteristic
feature of primary hypertension independent of obesity. Metabolism 1990;39:167-174
22. Modan M, Halkin H: Hyperinsulinemia or increased sympathetic
drive as links for obesity and hypertension. Diabetes Care 1991;14:
470-487
23. Tuck M: Treatment of hypertensive diabetic patients. Diabetes
Care 1988;ll:828-832
24. Houston MC: The effects of antihypertensive drugs on glucose
intolerance in hypertensive non-diabetics and diabetics. Am Heart
J 1988;115:640-656
25. Bengtsson C, Blohme G, Lapidus L, Lindquist O, Lundgren H,
Nystrom E, Petersen K, Sigurdsson JA: Do antihypertensive drugs
precipitate diabetes? BrMedJ 1984;289:1495-1497
26. Stein PP, Black HR: Drug treatment of hypertension in patients
with diabetes mellitus. Diabetes Care 1991;14:425-448
Kautzky-Willer et al
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
27. Landsberg L: Hyperinsulinemia: A possible role in obesity-induced
hypertension. Hypertension 1992;19(suppl I):I-61—1-66
28. Gupta AK, Clark RV, Kirchner KA: Effects of insulin on renal
sodium excretion. Hypertension 1992;19(suppl I):I-78-I-82
29. Singer DE, Nathan DN, Keaven MA, Wilson PWF, Evans JC:
Association of HbAlc with prevalent cardiovascular disease in the
original cohort of the Framingham Heart Study. Diabetes 1992;41:
202-208
30. Verdonk CA, Rizza RA, Gerich JE: Effects of plasma glucose
concentrations on glucose utilization and glucose clearance in normal man. Diabetes 1981;30:535-537
31. Bergman RN, Bucolo RJ: Interaction of insulin and glucose in the
control of hepatic glucose balance. Am J Physiol 1974;227:1314-1322
32. Bergman RN, Hope ID, Yang YJ, Watanabe RM, Meador MA,
Youn JH, Ader M: Assessment of insulin sensitivity in vivo: A critical
review. Diabetes Metab Rev 1989;5:411-429
33. Kautzky-Willer A, Pacini G, Ludvik B, Schernthaner G, Prager R:
Beta-cell hypersecretion and not reduced hepatic insulin extraction causes predominantly hyperinsulinemia in obesity. Metabolism
1992;41:1304-1312
34. Marchesini G, Pacini G, Bianchi G, Patrono D, Cobelli C: Glucose
disposal, beta cell secretion and hepatic insulin extraction in cirrhosis: A minimal model assessment. Gastroenterology 1990;99:
1715-1722
35. Baron AD, Brechtel G, Wallace P, Edelman SV: Rates and tissue
sites of non insulin and insulin mediated glucose uptake in humans.
Am J Physiol 1988;255:E769-E774
36. Polonsky K, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B: Use of biosynthetic human C-peptide
in the measurement of insulin secretion in normal volunteers and
type I diabetic patients. J Clin Invest 1986;77:98-105
37. Pacini G, Beccaro F, Valerio A, Nosadini R, Crepaldi G: Reduced
beta cell secretion and insulin hepatic extraction in healthy elderly
subjects. J Am Geriatr Soc 1990;38:1283-1289
38. Laasko M, Sarlund H, Mykkanen L: Essential hypertension and
insulin resistance in non-insulin-dependent diabetes. Eur J Clin
Invest 1989;19:518-526
Insulin Metabolism in Essential Hypertension
653
39. Pollare T, Lithel H, Berne C: A comparison of the effects of
hydrochlorothiazide and captopril on glucose and lipid metabolism
in patients with hypertension. N Engl J Med 1989;321:868-873
40. Kendall MJ, Horton RC, Chellingsworth MC: Calcium antagonists
and glycaemic control. J Clin Hasp Pharmacol 1986;11:175-180
41. Klauser R, Prager R, Gaube S, Gisinger C, Schnack C, Kiienburg
E, Schernthaner G: Metabolic effects of isradipine versus hydrochlorothiazide in diabetes mellitus. Hypertension 1991;17:15-21
42. Pollare T, Lithell H, Morlin C, Prantare H, Hvarfner A, Ljunghall
S: Metabolic effects of diltiazem and atenolol: Results from a
randomized, double-blind study with parallel groups. J Hypertens
1989;7:551-559
43. Ferrari P, Weidmann P: Insulin sensitivity in humans: Alterations
during drug administration and in essential hypertension. Miner
Electrolyte Metab 1990;16:16-24
44. Santoro D, Natali A, Palombo C, Brandi LS, Piatti M, Ghione S,
Ferrannini E: Effects of chronic angiotensin converting enzyme
inhibition on glucose tolerance and insulin sensitivity in essential
hypertension. Hypertension 1992;20:181-191
45. Fournier AM, Gedia MT, Kubrusly CB, Skyler JS, Sononko JM:
Blood pressure, insulin, and glycemia in nondiabetic subjects. Am
J Med 1986;80:861-864
46. Bonora B, Zavaroni I, Alpi O, Pezzarossa A, Bruschi F, Dall'Aglio
E, Guerra L, Coscelli C, Butturini U: Relationship between blood
pressure and plasma insulin in non-obese and obese non-diabetic
subjects. Diabetologia 1987;307:719-723
47. Manolio TA, Savage PJ, Burke GL, Liu KA, Wagen Knecht LE,
Sidney S, Jacobs DR Jr, Roseman JM, Donahue RP, Oberman A:
Association of fasting insulin with blood pressure and lipids in
young adults: The CARDIA study. Arteriosclerosis 1987;7:197-202
48. Mbanya JC, Thomas TH, Wilkinson R, Alberti KGMM, Taylor R:
Hypertension and hyperinsulinemia: A relation in diabetes but not
essential hypertension. Lancet 1988;l:733-744
49. Grugni G, Ardizzi A, Dubini A, Guzzaloni G, Sartorio A, Morabiti
F: No correlation between insulin levels and high blood pressure in
obese subjects. Horm Metab Res 1990;22:124-125
50. Julius S, Gudbrandsson T, Jamerson K, Shahab ST, Andersson O:
The hemodynamic link between insulin resistance and hypertension. Hypertension 1991;9:983-986
Elevated hepatic insulin extraction in essential hypertension.
A Kautzky-Willer, G Pacini, M Weissel, M Capek, B Ludvik and R Prager
Downloaded from http://hyper.ahajournals.org/ by guest on May 5, 2017
Hypertension. 1993;21:646-653
doi: 10.1161/01.HYP.21.5.646
Hypertension is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1993 American Heart Association, Inc. All rights reserved.
Print ISSN: 0194-911X. Online ISSN: 1524-4563
The online version of this article, along with updated information and services, is located on
the World Wide Web at:
http://hyper.ahajournals.org/content/21/5/646
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally
published in Hypertension can be obtained via RightsLink, a service of the Copyright Clearance Center,
not the Editorial Office. Once the online version of the published article for which permission is being
requested is located, click Request Permissions in the middle column of the Web page under Services.
Further information about this process is available in the Permissions and Rights Question and Answer
document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Hypertension is online at:
http://hyper.ahajournals.org//subscriptions/