* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Basic Physical Chemistry Lecture 1
Noether's theorem wikipedia , lookup
Double-slit experiment wikipedia , lookup
Perturbation theory wikipedia , lookup
Two-body Dirac equations wikipedia , lookup
Canonical quantization wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Spherical harmonics wikipedia , lookup
Atomic orbital wikipedia , lookup
Lattice Boltzmann methods wikipedia , lookup
Density matrix wikipedia , lookup
Coupled cluster wikipedia , lookup
Tight binding wikipedia , lookup
Particle in a box wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Path integral formulation wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Atomic theory wikipedia , lookup
Matter wave wikipedia , lookup
Renormalization group wikipedia , lookup
Probability amplitude wikipedia , lookup
Wave–particle duality wikipedia , lookup
Schrödinger equation wikipedia , lookup
Dirac equation wikipedia , lookup
Wave function wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
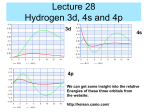
Basic Physical Chemistry Lecture 1 Keisuke Goda Summer Semester 2015 Lecture schedule Since we only have three lectures, let’s focus on a few important topics of quantum chemistry and structural chemistry Lecture 1 Hydrogen atom – The most basic unit of chemistry Lecture 2 Angular momentum (spin) – This is what makes chemistry interesting Lecture 3 Molecular structure – The origin of various unique properties of molecules Chapter 1: The Wave Function 1.1: The Schrodinger Equation 1.2: The Statistical Interpretation 1.3: Probability 1.4: Normalization 1.5: Momentum 1.6: The Uncertainty Principle Classical mechanics In classical mechanics, we use Newton’s second law [F = ma] to find the position [x(t)] F ma V d x m 2 x dt 2 Classical mechanics In classical mechanics, if we know the initial conditions of the particle, we can determine the final state of the particle (position & momentum) by using Newton’s second law [F = ma] Why not classical mechanics for small objects? Unfortunately, we cannot use classical mechanics to describe microscopic particles (atoms and molecules) Incorrect Correct Because microscopic particles exist probabilistically (we do not know their states until we measure them) Quantum mechanics The quantum-mechanical analog of Newton’s F = ma is the Schrodinger equation. Schrodinger Equation ᴪ = wave function 1. Quantum mechanics is all about the Schrodinger equation. 2. Our goal is to solve this equation for the wave function under various conditions. Erwin Schrodinger Schrodinger’s grave So, what is this Schrodinger equation?? Schrodinger Equation ᴪ = wave function 2 p E V 2m E i t p E V 2m 2 p i x The Schrodinger equation looks similar to the equation of energy conservation in classical mechanics, but not quite! Derivation of the Schrodinger equation (1) Let’s assume that is a wave function with wavenumber k and angular frequency . 0 ei kx t Then, let’s differentiate this wave function with respect to x. ik0 e i kx t ik x Differentiating this function one more time yields 2 2 i kx t 2 ik e k 0 2 x Using the de Broglie equation k 2 p p2 2 2 2 x On the other hand, the derivative of the wave function with respect to t is given by i0 e i kx t i t Using the Planck relation E iE t Derivation of the Schrodinger equation (2) The derivatives of the wave function can be rewritten as p2 2 2 2 x iE t 2 2 2 p x 2 E i t Now, let’s consider the energy equation which is the sum of the kinetic energy and the potential energy. p2 E 2m V Putting the wave function on both sides of the equation gives 2 p E V 2m Substituting E and p2 into this equation gives the Schrodinger equation. 2 2 i V 2 2m x t Operators Comparing the following two equations gives birth to the concept of “operators.” 2 p E V 2m E i t 2 2 V i 2 2m x t p i x • Never mind why the energy and momentum become operators. • We can do quantum mechanics if we accept this assumption. So, what is the wave function?? Born’s statistical interpretation of the wave function Collapse of the wave function Collapse of the wave function after a measurement Probability of continuous variables We have dealt with discrete variables, but it is simple enough to generalize the theory to continuous distributions. Probability density (probability of getting x) Probability that x lies between a and b Obvious relations The integration of the probability density over the entire range is 1 (of course). Average value of x Average value of f(x) Variance of x Need for normalization Remember Born’s statistical interpretation of the wave function Probability density for finding the particle at point x at time t The integration of the probability density over the entire range is 1 (of course again). Requirement for normalization Expectation value of position x Expectation value of x It does not mean that if you measure the position of one particle over and over again, <x> is the average of the results you will get. Interpretation of the expectation value The expectation value is the average of repeated measurements on an ensemble of identically prepared systems, not the average of repeated measurements on one and the same system. Expectation value of momentum p Take the derivative with respect to time Use integration by parts Use integration by parts again Let’s postulate that the expectation value of the velocity is the time derivative of the expectation value of the position (we will prove this in Chapter 3) More suggestive expressions of x and p • The operator • The operator x i x represents position represents momentum To obtain the expectation value of position x and momentum p, we sandwich each operator between ᴪ* and ᴪ and then integrate. Generalized expectation value Q = Q(x, p) = any function of x and p To obtain the expectation value of Q, we simply replace every p with the operator, insert the resulting operator between ᴪ* and ᴪ, and integrate. For example, the expectation value of the kinetic energy is given by T is a function of p Replace p with the operator in the integral Chapter 4: Quantum Mechanics in Three Dimensions 4.1: Schrodinger Equation in Spherical Coordinates 4.2: The Hydrogen Atom 4.3: Angular Momentum 4.4: Spin Generalization to 3D So far we have considered Schrodinger’s equation only in 1D, but it can be extended to 3D The momentum operator in each dimension is given by Schrodinger’s equation hence becomes where Schrodinger’s equation in 3D (This is called the Laplacian) Schrodinger’s equation in spherical coordinates In most cases in chemistry, the potential V is a function only of the distance from the origin, which means that V = V(r) = V(r, , ) Schrodinger’s equation in spherical coordinates Schrodinger’s equation in spherical coordinates Cartesian coordinates Spherical coordinates Schrodinger’s equation in spherical coordinates (in terms of r, , ) Separation of variables in spherical coordinates In order to solve the Schrodinger equation, we separate the wave function into the radial part, R(r), and the angular part, Y(, ) Putting this into the Schrodinger equation yields Simplifying this equation gives The first tem is only a function of r whereas the second term only depends on and , such that each term must be constant Separation of variables in spherical coordinates Constant Constant The constant is written in this form for some reason (Never mind this for now, but this substitution will turn out to be very convenient and meaningful and be explained in Chapter 4.3) Radial part Angular part Our next step is to solve each equation for R(r) and Y(, ) Angular part Again, the angular part is given by As always, we separate variables Putting this into the angular equation, we find The first tem is only a function of whereas the second term is only a function of , such that just like before, each term is written as a constant Never mind why we use the constant m2 for now (this will be explained in Chapter 4.3) Solving the equation The equation is easy to solve Since is the azimuthal angle, the solution also allows This means m must be an integer Solving the equation The equation is not so easy to solve The solution is given by where is called the associated Legendre function defined by and is called the Legendre polynomial defined by the Rodrigues formula Legendre polynomial The Legendre polynomial is even or odd, depending on the value of l Associated Legendre functions If m is odd, P is a function of sin If m is even, P is a function of cos Requirement for l and m Notice that l must be a non-negative integer for the Rodrigues formula to make any sense If For any given l, then there are , then possible values of m For example, if l = 0, m = 0; if l = 1, m = -1, 0, 1; if l = 2, m = -2, -1, 0, 1, 2; and so forth Normalization and solution The volume element in spherical coordinates is given by So the normalization condition becomes It is convenient to normalize R and Y separately The normalized angular wave functions are called spherical harmonics Orthogonality and quantum numbers Spherical harmonics The spherical harmonics are orthogonal (I will leave the proof to you) l is called the azimuthal quantum number and m is called the magnetic quantum number Radial part Notice that the angular part of the wave function, Y(, ), is the same for all spherically symmetric potentials whereas the actual shape of the potential, V(r), affects only the radial part of the wave function, R(r), which is given by This equation can be simplified if we use the substitution Radial equation This radial equation is identical in form to the 1D Schrodinger equation except for the effective potential Normalization of the radial wave function The normalization condition becomes since This looks like the normalization condition in 1D, but remember that because of this substitution, we have the extra term in the effective potential Effective potential So, the strategy for solving the equation is first to find u(r) and then obtain R(r) = u(r) / r Short summary of the 3D Schrodinger equation Schrodinger’s equation in spherical coordinates (in terms of r, , ) The function is separated into the radial part, R(r), and the angular part, Y(, ) The angular part is found to be The radial part can be solved for when V is provided Hydrogen atom Finally, we are getting closer to quantum “chemistry” based on our knowledge of quantum mechanics, but be warned that even the wave function of the simplest model (hydrogen atom) is not so simple Hydrogen atom Based on the Schrodinger equation in 3D spherical coordinates, we are ready to solve the radial part of the Schrodinger equation for hydrogen me In theory, we should use the reduced mass, but since mp is about 1000 times larger than me and we can replace the reduced mass with me Schrodinger’s equation for the hydrogen atom From Coulomb’s law, the potential energy (which only depends on r) is given by The radial equation is given by This equation allows both continuum states (E > 0), describing electron-proton scattering, as well as discrete bound states (E < 0), representing the hydrogen atom Solution to the radial equation Unfortunately, the calculation of this radial equation is too lengthy to cover in this lecture. Therefore, I will leave the calculation to you (it’s covered over several pages in Griffiths), and let me get straight to the answers directly. Hint: If you want to really solve this differential equation, focus on the first few values of l (l = 0, l = 1, etc.) and you can solve the equation. Radial wave functions for hydrogen Remember R(r) = u(r) / r Here a is the characteristic constant defined to be This is called the famous Bohr radius. Radial wave functions for hydrogen Normalized hydrogen wave functions Combining the radial and angular wave functions yields the normalized wave functions for the hydrogen atom where is called the associated Laguerre polynomial is called the Laguerre polynomial Normalized hydrogen wave functions The wave functions look quite ugly, but the hydrogen atom is one of the very few realistic systems that can be solved analytically (the Schrodinger equation in all other chemical systems needs to be solved numerically by using computer simulations) Just like in the previous examples (infinite square well and harmonic oscillator), wave functions with different (n, l, m) values are orthogonal The wave functions are mutually orthogonal Density plots for the hydrogen wave functions Surfaces of constant probability densities Probability densities for various orbitals Bohr formula Substitute the radial wave functions into the radial part of the Schrodinger equation Bohr formula Niels Bohr obtained this function in 1913 by using the Bohr model (a premature mixture of classical physics and quantum physics). Note that the Schrodinger equation did not come until 1924. Hydrogen energy levels in more detail The allowed energy levels for the hydrogen atom (without corrections) are given by A few major corrections (which can be obtained by using the perturbation theory) are given by Fine structure (relativistic correction + spin-orbit coupling) Hyperfine splitting (spin-spin coupling) Energies of the first few states The ground state (the state of the lowest energy) is the case n = 1 (hence, l = 0), and therefore, the ground state energy is given by If n = 2, the first excited state energy is given by Here l is either l = 0 (with m = 0) or l = 1 (with m = -1, 0, 1) Spectrum of hydrogen • In principle, if you put a hydrogen atom into some stationary state, it should stay there forever. • However, if you tickle it slightly (by collision with another atom or shining light on it), the electron may undergo a transition to some other stationary state – either by absorbing energy (absorbing a photon and moving up to a higher energy state) or by giving off energy (emitting a photon and moving down to a lower energy state). • This transition energy corresponds to the difference in energy between the initial and final states. Energy difference Initial state Final state Spectrum of hydrogen According to the Planck formula, the energy of a photon is proportional to its frequency Meanwhile, the wavelength is given by Combining these two equations, we find the so-called Rydberg formula where R is called the Rydberg constant Energy levels and transitions Lyman series Transitions to the ground state (n = 1) In the ultraviolet range Balmer series Transitions to the first excited state (n = 2) In the visible range Paschen series Transitions to the second excited state (n = 3) In the infrared range Energy levels and transitions Energy levels and transitions Spectroscopy Absorption spectra of various elements Quiz Answer the following questions about the hydrogen atom. The table of the radial wave functions for hydrogen on the right may be useful for your calculations. Provide your answers in terms of the Bohr radius ܽ. (a) Find the position of the electron in the 1s orbital where it is most likely to be found. (b) Find the position of the electron in the 2s orbital where it is most likely to be found. (c) Find the position of the electron in the 2p orbital where it is most likely to be found. (d) Find the position of the electron in the 3d orbital where it is most likely to be found. Second report • Read the paper “The Early History of Spectroscopy” by N. C. Thomas in Journal of Chemical Education 68, 631 (1991) and write a 1-page report about it in English. • The theme of your report is about how spectroscopy has been developed • The report should be written in a A4-sized paper. • Only single spacing is acceptable (no double spacing). • Font size: 11 pt • Font type: Arial, Times New Roman, or Calibri • Write down your name and ID number at the top of your report. • Do not forget to write the title for your report. • Submission deadline: 4/21 • Put your report on the lecturer’s desk before the lecture begins.