* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Free PDF - European Review for Medical and
Survey
Document related concepts
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Myocardial infarction wikipedia , lookup
Cardiac surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Echocardiography wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
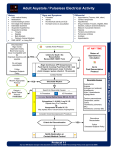
European Review for Medical and Pharmacological Sciences 2010; 14: 77-88 The proposal of an integrated ultrasonographic approach into the ALS algorithm for cardiac arrest: the PEA protocol A. TESTA1, G.A. CIBINEL2, G. PORTALE3, P. FORTE1, R. GIANNUZZI1, G. PIGNATARO1, N. GENTILONI SILVERI1 1 Department of Emergency Medicine, “A. Gemelli” University Hospital, Rome (Italy) Department of Emergency Medicine, General Hospital ASL To 3, Pinerolo, Turin (Italy) 3 Department of Emergency Medicine, “S. Antonio Abate” Hospital, Tolmezzo, Udine (Italy) 2 Abstract. – Background and Objective: Guidelines on cardiac arrest (CA) recommend the prompt beginning of cardio-pulmonary resuscitation (CPR) and the identification and correction of reversible causes. This article deals with the application of clinical ultrasonography (US) in resuscitation, presenting a simple codified US protocol usable during CPR to recognize reversible causes of CA. Evidence on US in CA and State of the Art: Emergency US is a bedside, point-of-care, focused diagnostic procedure with aim to complete the physical examination. It is performed by emergency physician everywhere to answer briefly important clinical questions. Several trials recently experimented US employment during advanced life support, demonstrating its feasibility without delaying CPR. Perspectives: the PEA Protocol:: We propose a simplified US protocol for non-shockable rhythms, called “PEA protocol” to remember the applications of the study (CA in Pulseless Electrical Activity, PEA) and the US scan sequence: Pulmonary scans to depict pneumothorax and pleural effusion and to differentiate wet or dry lung; Epigastric for pericardial effusion, left and right ventricular sides and motion, IVC filling; Abdominal and other scans for aortic aneurism and dissection, peritoneal effusion, bowel occlusion or perforation, deep venous thrombosis. The PEA protocol could be performed both during CA in PEA and during periarrest conditions. Conclusions: Clinical US, using a well codified protocol, could effectively help to identify reversible causes in CA, even improving patients outcome. Key Words: Ultrasound, Cardiac arrest, Cardio pulmonary resuscitation, Advanced life support, Hypovolemia, Tamponade, Pneumothorax, Pulseless electrical activity. Introduction Out-of-hospital incidence of cardiac arrest (CA) in Europe, from the activation of the medical emergency team (MET), is about 4:10,000 per year, while in-hospital incidence of CA is a little less than 4:1000 per year, that is one case each 6 beds, with an extra-/in-hospital ratio about 1:101,2. The mean survival to hospital discharge from a CA in shockable rhythms is estimated about 20%1,3. Instead, the survival from an in-hospital CA from ventricular fibrillation (VF) pulseless/persistent ventricular tachicardia (VT), or from non-shockable rhythms as inset (Asystole and Pulseless Electrical Activity, PEA), decreases in mean below 10%2-6, and falls down 3% in cases of out-of-hospital CA from VF/pulseless-persistent VT or non-shockable rhythms1. Interventions that could improve the prognosis of a CA are synthesized in the so-called “chain of survival”, including: (1) prevention and early recognition of CA; (2) early cardio-pulmonary resuscitation (CPR); (3) attempt defibrillation if appropriate (VF/pulseless VT); (4) post-resuscitation care7. CA prognosis has actually improved after the introduction and the employment of ACLS (Advanced Cardiac Life Support) protocols, worked out by American Heart Association (AHA)8 and ALS (Advanced Life Support) protocols, formulated by European Resuscitation Council (ERC)9. During the management of shockable rhythms (VF/pulseless VT) prognosis has significantly improved after the introduction of defibrillation; it is not the same in the management of non-shockable rhythms (Asystole/PEA)4. The prompt beginning of thoracic compressions with minimal interruption of cardio-pulmonary resuscitation (CPR) to reduce the no-flow Corresponding Author: Americo Testa, MD; e-mail: [email protected] 77 A. Testa, G.A. Cibinel, G. Portale, P. Forte, R. Giannuzzi, G. Pignataro, N. Gentiloni Silveri intervals and the identification and correction of underlying reversible causes, in order to reduce mortality, are strongly recommended by all international guidelines for resuscitation, which establish common guidelines for management and treatment of CA and peri-arrest9-11. The potentially reversible causes are suggested to be easily recalled by “4Hs” and “4Ts” sequence: Hypoxia, Hypovolemia, Hypothermia, Hypo/Hyperkalemia; cardiac Tamponade, tension pneumoThorax, coronary or pulmonary Thrombosis, Toxins and drugs. To detect the potentially reversible causes of CA, patient’s history could help. Unfortunately, these data are not always easily collected in emergency setting. An accurate physical examination, reading the arterial blood test and the monitoring of the most important vital parameters (Table I), could help the diagnosis too, while common blood tests are not always available or obtained in a few time. Ultrasonography (US) performed bedside during resuscitation could aid the diagnosis in four of the commonest reversible causes of CA, except for hypoxia: hypovolemia, cardiac tamponade, tension pneumothorax and thrombosis12-14. Such imaging information could change team leader’s therapeutic strategy and guide the management of the patients15. So we could underline the role of US in ruling in or ruling out the reversible causes of PEA, in order to reduce mortality and to improve the outcome, hoping for balance the role of DC shock in the algorithm of CA (Figure 1). Unfortunately, although the interest of US in acute resuscitation is widely recognized and its use strongly recommended, the modality for achieving as much as possible complete evaluation remains poorly codified. In this article we propose a simplified but nearly complete and well codified US approach, that we named “PEA protocol”. It consists in easily memorized US focused sequence that should be performed during CPR, to provide a thorough cardio-pulmonary assessment in order to recognize the commonest underlying causes of CA. Emergency US in Cardiac Arrest During the last few years, the diagnostic employment of clinical US diffused widely in Emergency Department (ED), since it provides a bedside, rapid, accurate, non invasive, painless and low cost exam playing an ever-growing role in multiple different diseases, so much to imply the idea of a “real US revolution” in medicine16. Emergency US should be considered a goal-directed focused examination complementary to the physical examination. It is performed by emergency physician in any emergency setting to answer brief and important clinical questions. Emergency US is a first level diagnostic tool and it should not quarrel with traditional consultative ultrasound imaging. The Focused Assessment with Sonography for Trauma (FAST) is now accepted in ATLS guidelines for management of trauma by American College of Surgeons Committee on Trauma17. On the other hand, scientific evidence (Class I) exists for FAST examination and for ultrasound-assisted central ve- Table I. The potentially reversible causes of cardiac arrest (CA), to be investigated and treated during interruption of cardio-pulmonary resuscitation (CPR), according to Advanced Life Support (ALS) guidelines by European Resuscitation Council (ERC). Cause Clinical findings (beside history) Treatment 4 “Hs” 1. Hypovolemia 2. Hypoxia 3. Hypothermia 4. Hyper-hypokaliemia Empty external jugular veins Cyanosis, BGA, airway obstruction Low body temperature BGA Fluid/blood infusion Opening, removal, ventilation Active/passive warming Electrolytes imbalance and metabolic disorders correction Fill jugulars, pulseless to CPR Thrombolisis, embolectomy, primary angioplasty (PTCA) Pericardiocentesis Drainage by 14G needle Gastrolavage, charcoal, antidots/antagonists 4 “Ts” 1. Pulmonary or coronaric Thromboembolism 2. Cardiac Tamponade 3. Tension Pneumo-Thorax 4. Toxic and drugs Legends: BGA = blood gas analysis. 78 Fill jugulars, pulseless to CPR Fill jugulars, pulseless to CPR Bradycardia, neurologic examination, pupils Ultrasound in cardiac arrest Figure 1. Proposal of introduction of US for non-defibrillable rhythms arm in the ALS algorithm for Cardiac Arrest to balance the DC shock for defibrillable rhythms arm. nous catetherism18. Therefore, US can be applied to any emergency medical condition with diagnostic, therapeutic and monitoring fashion, including acute resuscitation19. Actually, most causes of PEA are referable to specific echocardiografic findings. On 1997 Varriale and Maldonaldo’s study design12 for in-hospital CA included a dedicated team of cardiologists to perform transthoracic US examination by subcostal or apical view or, if not possible, trans-oesophageal echocardiography. This study concluded that US during CPR was not only feasible but provided useful information in management of CA. Leading causes of CA could be addressed, with potential depiction among the causes of pseudo-electromechanical dissociations: pulmonary embolism, cardiac tamponade and hypovolemia. In the wake of the FAST protocol efficacy, different Authors have then formulated other acronyms in order to define sonographic protocols that could be performed to critical patients, either with or without trauma. In the EFAST pro- tocol the detection of pneumothorax is added20-22. Successively, the FAST-CRASH extends sonographic employment even in the CA and peri-arrest conditions23. Finally, the FAST-ABCDE includes emergency US in each phase of the primary survey ABCDE of trauma patients, with diagnostic aims and as help in invasive procedures24. In 2004, Jensen et al13 worked out firstly a rapid echocardiographic protocol targeted for critical patient, formulating the “mystic” acronym FATE (“Focus Assessed Transthoracic Echo protocol”), demonstrating its feasibility in 97% of cases, even if performed by not cardiologist. In 2005, Niendorff et al25 studied a wide consecutive series of hospitalized patients who underwent PEA arrest and concluded that a rapid US examination (though performed by nonexpert ultrasonographers) could be successfully integrated into ACLS response system. The average time from arrest alert to US interpretation was ≤8 minutes. The duration of interruption occurring was not longer than the time necessary for pulse check. The opti79 A. Testa, G.A. Cibinel, G. Portale, P. Forte, R. Giannuzzi, G. Pignataro, N. Gentiloni Silveri mal windows resulted the subcostal or apical views because minimized the delay for compressions. In 2007, Breitkreutz et al26 strengthened that such technique, inserted during the pauses for pulse check or during compression in out-of-hospital patients, didn’t hinder the common CPR manoeuvres. The Authors formulated a fast protocol, called with the acronym FEER (Focused Echocardiographic Evaluation in Resuscitation management), including 3 standard scans that could be used in the study of the heart during CPR. In 2008, the “etiologic” acronym CAUSE (Cardiac Arrest UltraSound Examen) was suggested. It got away from the mere “cardiologic perspective” adding to the study of the heart even the sonographic evaluation of the lung, for the detection of tension pneumothorax. Both of them were performed using the convex transducer14. Always in 2008, Cibinel et al27 demonstrated, in a series of 30 patients, the feasibility of cardiopulmonary sonography in 100% of CA and peri-arrest, again ruling out potential interference with CPR manoeuvres. US diagnostic and therapeutic impact was also validated. Most recently we proposed a simplified US protocol19 that can be easily inserted in the common ALS algorithm for non-shockable rhythms, called with the “mnemonic” acronym “PEA protocol”. Such protocol’s name may help to remember either the applications of the study (CA in PEA) or the US scan sequence to be performed (“P” for Parasternal, “E” for Epigastric and “A” for Abdomen and other scans). PEA Protocol Definition The revised PEA protocol we propose in this review consists of a codified 3 scan sequence of US views to be integrated into ALS algorithm during CPR, in order to recognize underlying reversible causes of CA. According to previous reports 14,25 the beginning by cardiac imaging, a “black box” in most cases of CA26, is recommended for obtaining the most important critical findings on pericardium cavity and myocardial sides and motion (main scans). Then, the other pleuro-pulmonary (complementary scans), thoracic abdominal and peripheral scans (additional scans) are carried on in agreement with the suspected diagnosis based on patient’s history, clinical data and just obtained focused cardiac US findings (Figure 2): 80 P = Pulmonary scans, for research of pneumothorax, pleural effusion, wet or dry lung; E = Epigastric and other scans, for research into pericardial effusion, left and right ventricular sides and their wall motion and inferior vena cava (IVC) filling; A = Abdominal and other scans, for research into thoracic and aortic aneurism and/or dissection, peritoneal effusion, bowel occlusion or perforation, deep venous thrombosis. US Scans Sequence in the PEA Protocol The PEA protocol could be performed both during CA in PEA and during peri-arrest conditions, other than after the recover of a spontaneous rhythm from defibrillation, to check the treatable causes if PEA is not justified by hypoxia or electrolyte abnormalities, toxaemia and hypothermia. The sequence of the 3 codified US views in PEA protocol may change based on the patient’s condition. Cardiac Arrest in PEA The pulmonary (P) scans may be difficult to interpret in detecting pneumothorax in artificially ventilated patients in CA by the self-inflating bag, because of limited lung sliding. However, these scans could be useful after intubation to exclude pneumothorax other than to control the correct position of the tube. The finding of motionless US lung comets (ULCs)28 or B lines29, like the glare of the sun on the water, can distinguish a wrong intubation from a pneumothorax, in which there could never be find ULCs19. Therefore, in the CA firstly the cardiac evaluation by performing epigastric and other scans (E) is recommended (main scans), the US scans used subsequently varying according to the initial findings. Other than pericardial effusion and inferior vena cava filling, heart motion is the main US picture to research14,26. Heart wall movement with pulse means presence of circulation. Absence of wall movement means asystole or true PEA (if a rhythm is present on monitoring): this condition represents usually a final phase of any cause of CA. If a hypokinetic, enlarged or hypertrophic left ventricle is detected, the pulmonary scans (P) are preferred to depict a wet lung, characterized by the interstitial-alveolar syndrome and pleural effusion29. If a hypokinetic and enlarged right ventricle is detected, pulmonary embolism is suspected: the pulmonary scans can reveal a dry lung and venous compression US (CUS) of lower limbs could be assessed to depict deep venous Ultrasound in cardiac arrest Pulmonary scans (Pneumothorax, pleural effusion, wet or dry lung) Abdominal and other scans (Aorta, bowel occlusion, abdominal effusion, DVT) Epigastric and other scans (Tamponade, IVC, heart sides and motion) Figure 2. PEA Protocol scheme is an easily remembering US sequence, consisting in 3 US focused scan approach: P = Pulmonary for pleural and pulmonary affections (blue), E = Epigastric and other scans for pericardium, heart, inferior vena cava (IVC) (red), and A = Abdominal and other scans for thoracic or abdominal aortic aneurism or dissection, abdominal effusion, bowel occlusion and/or perforation, deep venous thrombosis (DVT) (green). The team leader may each time choose to make the temporal sequence fit clinical signs or technical necessities. However, we recommend beginning with the E = Epigastric and the other cardiac views (main scans) to obtain maximal information about reversible causes of cardiac arrest, then carrying on P = Pulmonary (complementary scans) and A = Abdominal (additional scans) US examination, according to preliminary cardiac US findings. thrombosis. Presence of wall motion, pulseless and electrical activity means a pseudo-PEA (hypovolemia, cardiac tamponade, tension pneumothorax). If hypovolemia is suspected, the abdominal scans (A) should be then carried out to find potentially underlying pathologies (peritoneal effusion, abdominal aortic aneurism, bowel occlusion or perforation). Finally, if tamponade is found, US examination should be completed by the thoracic parasternal or suprasternal scan to depict potential aortic dissection. Several studies confirmed that US examination didn’t delay the normal CPR manoeuvres. Niendorff et al25 found that the average total CPR pause to US lasted less than 20 seconds and Breitkreutz et al26 added that such time for US examination decreased after training. Peri-arrest Conditions In the peri-arrest conditions, instead, the sequence scan could be performed from the beginning, following the order codified in the PEA protocol (Pulmonary-Epigastric-Abdomen), as it should be done in the EFAST. Moreover, the US examination can be carried out fitful concurrent- ly to the ABCDE evaluation, with the aim to depict and treat the potential causes each time they are encountered; in this case the performance of all scans is not necessary24. In the end, if there is a specific clinical suspicious, a focused approach is advised, beginning just from the site of the diagnostic doubt (lung in case of suspected pneumothorax, or abdomen for abdominal aortic aneurism, or performing a CUS for a suspected pulmonary embolism), then proceeding according to the first findings. In peri-arrest setting, moreover, a more complete and accurate evaluation is possible: for example on pulmonary scans a pneumonia or an empyema can be investigated in a dyspnoeic and/or septic patient. Furthermore, other than thoracic even septic cardiac foci could be investigated (endocarditis) and abdominal (free or saccate peritoneal fluid collection, organ abscesses, hydronephrosis)19. Tecnique and US Findings P = Pulmonary Scans What about the lung? The parasternal window, performed by a convex transducer put longitudinally in the second or third intercostal space, ad81 A. Testa, G.A. Cibinel, G. Portale, P. Forte, R. Giannuzzi, G. Pignataro, N. Gentiloni Silveri jacent to the sternum regulating the depth very low (6-8 cm), can easily exclude or confirm the diagnosis of pneumothorax. Several studies have demonstrated the accuracy of US in detecting PNX30,31. Three US findings should be searched: (1) the “gliding (or sliding) sign” whose presence allows to exclude almost absolutely pneumothorax and whose absence allows to confirm it with high accuracy (possible false positive results include fibrothorax, pneumonectomy, talc pleurodesis, wrong intubation, lung fibrosis, lung consolidations, acute respiratory distress syndrome (ARDS) 30; (2) ultrasound lung comets (ULCs)28 or B lines29, longitudinal artifacts arising from the pleural line and moving consensually to the gliding: ULCs are commonly detectable in healthy people, therefore they are wanting in clinical relevance. Even just one ULC allows, however, to rule out pneumothorax, while the absence of ULCs itself has not a diagnostic value. Many ULCs or even confluent depict interstitialalveolar syndrome (“wet lung”), typical of pulmonary oedema, ARDS, pneumonia, lung contusion and other interstitial pathologies; (3) the anechoic fluid collection in specific areas, limited by anterior chest wall and hemidiaphragm with lower lung lobe floating inside it32. Other than the diagnosis of pneumothorax, chest scans are so a natural conclusion of cardiac examination (complementary scans). The detection of a wet lung, exactly, gives information about a left ventricle (LV) failure, worsened by an acute coronary syndrome (LV motionless), a deterioration of dilated cardiomiopathy (wide and quite still LV) or a diastolic failure (hypertrophic wall of LV). A dry lung, however, does not exclude other global obstructive causes (cardiac tamponade) or against right sections (pulmonary embolism and tension pneumothorax) and not obstructive one (hypovolemia)14,32. Lung evaluation of the basis performed by coronal scans, finally, provides information on the presence of pleural effusion. A massive pleural effusion (traumatic, iatrogenic or neoplastic hemothorax) could be a potential cause of hypovolemia; instead if it is small and bilateral, it could be associated to wet lung in LV failure. E = Epigastric and Other Scans Principal heart view in emergency setting is subcostal long axis, with extension to longitudinal IVC view by transducer movement on the right side and rotation of the probe. Alternative 82 views include apical scan on 5th intercostal space on midclavicular or midaxillary line, using the phased array transducer, regulating the depth to high values (16-20 cm), looking for the standardized US 4 or 5 chambers view. Five US findings should be investigated: (1) pericardial effusion with cardiac tamponade US signs (sistolic right atrium and/or diastolic right ventricle collapse); (2) global pump function, resulting absent or severely hypokinetic (true PEA) or hyperkinetic (pseudo-PEA); (3) left ventricle chamber (enlarged or empty), wall dimension (hypotrophic in systolic heart failure or hypertrophic in diastolic heart failure) and its motion (ischemic damage): such finding, effective in peri-arrest and in post ROSC setting, results non-specific during CPR because of myocardial “stunning”; (4) right ventricle enlargement (≥ left ventricle) and hypokinesy (McConnell sign), suggestive of massive pulmonary embolism, even though non-specific (chronic cor pulmonale)14,25,26; (5) IVC filling: its diameter should be measured just upstream of the origin of the hepatic veins, both in two-dimensional mode and in M-Mode33,34. A full IVC (>20-25 mm), corresponding to a high right atrium pressure, could be detected, other than in motionless heart, in cardiac pump failure and in the so-called obstructive causes of CA, such as cardiac tamponade, pulmonary embolism and tension pneumothorax. A collapsed or flat IVC (<5 mm) is expression of hypovolemia and is connected with fluid responsiveness of the patient35. In patients not mechanically ventilated (peri-arrest for example, or shocked patients in spontaneous breathing) the values of filling are well correlated with right atrium pressure too, as demonstrated from the study of Kircher36, the maximum diameter of IVC being during expiration in spontaneous breathing. Manifest modifications of valves could complete the evaluation of cardiac dynamics. A = Abdominal and Other Scans This third group of US scans in PEA protocol are carried out using phased array, convex or linear transducer, depending on whether thorax, abdomen or the lower limbs, respectively, should be studied (Figure 2). The left parasternal scan on the 3rd or 4th intercostal space could be asked to look for a dissection of thoracic aorta or of a quite broken aneurism as cause of pericardial effusion, in the aim to indentify patients who need heart-surgery. Ultrasound in cardiac arrest In case of cardiac US findings suggestive of hypovolemia, abdomen deserves an accurate evaluation to research free blood or fluid collection, by broken abdominal aortic aneurism or of an other bowel artery, ectopic pregnancy, bowel occlusion and/or perforation. There is not a single position: (a) the upper lateral abdominal regions and pelvis should be investigated to explore the most dependent peritoneal site (Morison’s pouch) and the other principal spaces (perisplenic and pelvic), and the genito-urinary tract; (b) the epigastrium for the study of abdominal aorta; (c) the mesogastrium for bowel loops, eventually taut and for the research of fluid among loops. Finally the evaluation of kidney and urinary tract should not be forgotten in order to early detect an obstructive renal failure, potentially cause of hyperkaliemia, of acute heart failure and urinary sepsis. Moreover, supplemental US scans can be useful to detect deep venous thrombosis by compression US (CUS) on the lower limbs 37. Even if time-consuming, these peripheral US scans (abdominal and lower limbs) can be performed even during CPR, without preventing resuscitation manoeuvres. Main Recognized US Pictures The potential US findings during CPR are summarized above and the relative US images showed in Figure 3. Diagnostic conclusion and relative treatment are also presented. Asystole Absence of wall movement means true PEA, if a rhythm is present on monitoring. From data currently available in literature should derive a diagnostic accuracy of US near 100% in detecting a cardiac finding of absolutely absence of heart motion 38. If such considerations will be confirmed, the finding of a motionless heart during CPR manoeuvres could represent the indication for the interruption of further useless attempts39. Myocardial Insufficiency A severe failure of LV systolic function is suggested by a global or segmental wall motion reduction. The presence of a wide LV hypokinesia, especially in a peri-arrest setting or at the most at the beginning of CPR, connected to a lung pattern of interstitial-alveolar syndrome with or without bilateral pleural effusion and a flat IVC, let think of an acute coronary syndrome or of a worsening of heart failure. The presence at the same time of a compensatory hyperkinesia of some segments not involved into the ischemic event suggests however an acute occurrence. On the other hand, the maintenance of a normal myocardial kynesis in the doubt of an acute ischemic coronary event, would exclude almost completely an acute myocardial infarction40. A severe left ventricle hypertrophic wall means the possibility of a diastolic heart failure, especially if linked to a fill IVC and wet lung. Hypovolemia Presence of heart wall motion, pulseless and electrical activity means a pseudo-PEA. A low LV telediastolic volume, even detected from a rough macroscopic evaluation, if added to a low RV volume, represents itself an accurate parameter of a hypovolemic condition 41. The absence of a lung interstitial-alveolar syndrome (dry lung) and of pleural effusion consolidates the diagnosis of hypovolemia. The presentation begins standard if there is an empty IVC: in a peri-arrest or CA setting at the beginning of CPR, an IVC <5 mm or completely collapsed should be considered a sufficient parameter to begin an aggressive fluid resuscitation by crystalloid and colloid14,35. US exploration could be extended, as soon as possible, by peripheric chest and abdominal scans to search potential blood (hemothorax, hemoperitoneum) or fluid collection (bowel occlusion/perforation) and to detect possible occult hemorrhagic source (rupture of abdominal aortic aneurism or ectopic pregnancy). Cardiac Tamponade A pericardial effusion with US signs of tamponade, “dry lung” and a fill IVC depict the case of cardiac tamponade, accurate sonographic finding in every phase of CA and peri-arrest, even if performed by non-expert physicians42,43. The external jugular vein filling and pulseless during CPR represent non-specific clinical signs, detectable for example even in pulmonary embolism and in tension pneumothorax. Beside the high diagnostic accuracy, US could be really helpful as guidance in the following therapeutic 83 A. Testa, G.A. Cibinel, G. Portale, P. Forte, R. Giannuzzi, G. Pignataro, N. Gentiloni Silveri Figure 3. Main US pictures quickly recognizable by application of PEA protocol, beginning from cardiac findings (E = epigastric) and going on with complementary thoracic (P = pulmonary) and/or other additional US views (A=Abdomen and other scans), during CPR in CA, and corresponding diagnoses and consistent therapeutic decisions. Legends: CPR = cardiopulmonary resuscitation; CA = cardiac arrest; ULCs = ultrasound lung comets; IVC = inferior vena cava; DVT = deep venous thrombosis; PEA = pulseless electrical activity; PTCA = percutaneous transluminal coronary angioplasty. 84 Ultrasound in cardiac arrest procedure of pericardiocentesis: you should sting where you can see fluid collection on US scan. A hemopericardium with cardiac tamponade could represent the first sign of an aortic rupture or dissection: heart-surgery is the only therapeutic and final chance and so the early detection is highly advisable. Extending US evaluation to the great vessels origin by parasternal and eventually suprasternal scans, though with low sensibility, could result however basic, if transesophageal approach is not available. Pulmonary Embolism Enlarged right ventricle and fill IVC mean pulmonary hypertension (pulmonary embolism?). A negative chest US finding (dry lung) does not rule out pulmonary embolism, therefore concurrent CUS of lower limb veins is required to confirm diagnosis. However, studies showed that diagnosis of pulmonary embolism by chest US can actually be supported by depiction of wedge-shaped hypoechoic homogeneous pleural based lesions, suggesting early stages of pulmonary infarction44. Transthoracic US has been widely evaluated in the complex diagnosis of pulmonary embolism, even besides CA and peri-arrest settings, with sensibility evaluation about 50% and of specificity about 90%45,46. Diagnostic accuracy of such technique rises proportionally to the importance of embolism, where the major hemodinamic compromission is connected to a bigger risk of CA47. The end of the cardio-pulmonary US examination by performing a lower limb CUS in order to research deep venous thrombosis allows to confirm the diagnosis of pulmonary embolism in 39% of cases, with a specificity of 99%48. Pulmonary embolism accounts for cause of CA on the whole in 5% of cases49, but such prevalence reaches 36% of PEA cases50. In other retrospective autoptic studies in case of CA from pulmonary embolism, shockable rhythms have a prevalence of 5%, while the remaining 95% concerns non shockable rhythms, with a prevalence of PEA (63%) over asystole (32%)49. Thrombolysis efficacy seems corroborated by clinical trials and could represent a critical point in the research of an improvement in PEA outcome. Tension Pneumothorax In tension pneumothorax heart finding is essentially normal or small and hyperkinetic, IVC is fill but above all the “gliding sign” is missing in the affected side explored on parasternal scan30. Sometimes iatrogenic, or subsequent to an attempt of central catheter positioning or due to artificial ventilation in a patient with small pneumothorax, tension pneumothorax or “valve pneumothorax” represents a promptly reversible cause if recognised and managed by thoracentesis by needle and then by drainage. Diagnosis is above all clinic, suggested by high tympanites, reduction of vesicular respiration, opposite deviation of trachea and jugular swelling (Table I), but this is not easy detectable in the caotic setting of the Emergency Room. Pathogenesis is typically referable to atrio-caval junction torsion, with consequent right chambers repletion lack, which is the cause of CA. However, recent indications from animal experiments and human experiences ascribe the primary pathogenetic role moreover to hypoxia due to the wide right-left shunt in not ventilated lung: on hypoxia would depend the respiratory center damage and then the respiratory arrest. Hemodinamic parameters, instead, would be poor modified, at least in an early phase: CA arrest would represent, so, a late event and follows respiratory arrest51. Conclusion The widespread use of bedside emergency US is the direct consequence of a series of related events, all directed towards improving patient management at the point of care. Advances in technology have led to smaller, portable and easier to use US equipment with increasingly better image quality, specifically designed for Emergency Medicine. On the other hand, the increasing emphasis on patient safety, quality care, efficiency, less invasive treatment and non-ionizing imaging have found a natural fit with the advantages of US. Developed Countries have rapidly adopted emergency US and developing Nations naturally supplant other traditional and more expensive diagnostic techniques with US. The emergency US is now considered an essential skill in the practice of Emergency Medicine52. Many fellowship programs now exist worldwide, providing basic and advanced education and training for emergency physicians. Moreover, in some scientific communities the US education needs to be structured to allow emergency medicine residents to incorporate US into their daily clinical practice18. 85 A. Testa, G.A. Cibinel, G. Portale, P. Forte, R. Giannuzzi, G. Pignataro, N. Gentiloni Silveri Large randomized controlled trials have to be performed to complete an assessment of the scientific evidence on the effectiveness of emergency US in acute resuscitation. In our opinion, the universal ALS algorithm could update by technology, adding in the non-shockable rhythm branch US aid, as the shockable branch has already gained defibrillator (Figure 1). Clinical US with a simplified and codified protocol, like PEA protocol, could be early feasible as complimentary to the physical examination in the assessment of CA during most circumstances and sites. As recently proposed15, we hope the “chain of survival” could be early enriched by inserting a “fifth circle” to identify and treat reversible causes of CA. References 1) ATWOOD C, EISENBERG MS, HERLITZ J, REA TD. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 2005; 67: 75-80. 8) 2005 AMERICAN HEART ASSOCIATION GUIDELINES FOR CARDIOPULMONARY RESUSCITATION AND EMERGENCY CARDIOVASCULAR CARE. Circulation 2005; 112 (Suppl 1): 12-18. 9) NOLAN JP, DEAKIN CD, SOAR J, BOTTIGER BW, SMITH G. European Resuscitation Council guidelines for Resuscitation 2005. Resuscitation 2005; 67 (Suppl. 1): 39-86. 10) HAZINSKI MF, NADKARNI VM, HICKEY RW, O'CONNOR R, BECKER LB, ZARITSKY A. Major changes in the 2005 AHA guidelines for CPR and ECC: reaching the tipping point for change. Circulation 2005; 112 (Suppl. 24): IV206-211. 11) INTERNATIONAL LIAISON COMMITTEE ON RESUSCITATION. 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with treatment recommendations: Parts 1-8. Resuscitation 2005; 67: 181-314. 12) VARRIALE P, MALDONALDO J. Echocardiographic observations during in hospital cardiopulmonary resuscitation. Crit Care Med 1997; 25: 1717-1720. 13) J ENSEN MB, S LOTH E, L ARSEN KM, S CHMIDT MB. Transthoracic echocardiography for cardiopulmonar y monitoring in intensive care. Eur J Anaesthesiol 2004; 21: 700-707. 2) SANDRONI C, NOLAN J, CAVALLARO F, ANTONELLI M. Inhospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med 2007; 33: 237-245. 14) HERNANDEZ C, SHULER K, HANNAN H, SONYIKA C, LIKOUREZOS A, MARSHALL J. C.A.U.S.E.: Cardiac arrest ultra-sound exam–a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation 2008; 76: 198-206. 3) BRINDLEY PG, MARKLAND DM, MAYERS I, KUTSOGIANNIS DJ. Predictors of servival following in-hospital adult cardiopulmonary resuscitation. Can Med Assoc J 2002; 167: 343-348. 15) SLOTH E, JACOBSEN CJ, MELSEN NC, RAVN HB. The resuscitation guidelines in force-time for improvement towards causal therapy? Resuscitation 2007; 74: 198-199. 4) PEBERDY MA, KAYE W, ORNATO JP, LARKIN GL, NADKARNI V, M ANCINI ME, B ERG RA, N ICHOL G, L ANE T R U LT T T. Cardiopulmonar y resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 2003; 58: 297-308. 16) BAUMGARTEN RK. The real ultrasound revolution. Anesth Analg 2007; 104: 1292. 5) NADKARNI VM, LARKIN GL, PEBERDY MA, CAREY SM, KAYE W, MANCINI ME, NICHOL G, LANE-TRUITT T, POTTS J, ORNATO JP, BERG RA; NATIONAL REGISTRY OF CARDIOPULMONARY RESUSCITATION INVESTIGATORS. First documented rhythm and clinical outcome from inhospital cardiac arrest among children and adults. JAMA 2006; 295: 50-57. 6) M ENTZELOPOULOS SD, Z AKYNTHINOS SG, TZOUFI M, KATSIOS N, PAPASTYLIANOU A, GKISIOTI S, STATHOPOULOS A, K OLLINTZA A, S TAMATAKI E, R OUSSOS C. Vasopressin, epinephrine, and corticosteroids for inhospital cardiac arrest. Arch Intern Med 2009; 169: 15-24. 7) NOLAN J. European Resuscitation Council guidelines for resuscitation 2005. Section 1. Introduction. Resuscitation 2005; 67 (Suppl. 1): 3-6. 86 17) AMERICAN COLLEGE OF SURGEONS COMMITTEE ON TRAUMA. Advanced Trauma Life Support. 7th Edition, 2006. 18) AMERICAN COLLEGE OF EMERGENCY PHYSICIANS. ACEP POLICY STATEMENTS. Emegency ultrasound guidelines 2008, pagg. 1-38 (www.acep.org). 19) TESTA A. Manuale di Ecografia Clinica in Urgenza. Roma, Verduci Editore, 2008. 20) KIRKPATRICK AW, SIROIS M, LAUPLAND KB, LIU D, ROWAN K, B ALL CG, H AMEED SM, B ROWN R, S IMONS R, DULCHAVSKY SA, HAMIILTON DR, NICOLAOU S. Handheld thoracic sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma 2004; 57: 288-295. 21) SOLDATI G, TESTA A, SHER S, PIGNATARO G, LA SALA M, SILVERI NG. Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the Emergency Department. Chest 2008; 133: 204211. Ultrasound in cardiac arrest 22) TESTA A, SOLDATI G, PORTALE G, PIGNATARO G, GIANNUZZI R, GENTILONI SILVERI N. L’evoluzione della FAST nel politrauma: la Extended-FAST (o EFAST). Emergency Care Journal 2009 (in press). 23) CIBINEL GA. Ecografia clinica in emergenza-urgenza. Torino, C.G. Edizioni Medico Scientifiche, 2005. 24) NERI L, STORTI E, LICHTENSTEIN D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med 2007; 35 (Suppl): 290-304. 25) NIENDORFF DF, RASSIAS AJ, PALAC R, BEACH ML, COSTA S, GREENBERG M. Rapid cardiac ultrasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation 2005; 67: 81-87. 26) B REITKREUTZ R, WALCHER F, S EEGER FH. Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med 2007; 35(5 Suppl): S150-S161. 27) CIBINEL GA, SCALVENZO I, DESIDERIO S, MARTINI A, CASOLI G. Impatto diagnostico e terapeutico dell’ecografia integrata nei pazienti in arresto cardiorespiratorio e in periarresto. VI Congresso Nazionale SIMEU. Rimini, 2008: Nov 12-16, Atti pp.141-142. 28) PICANO E, FRASSI F, AGRICOLA E, GLIGOROVA S, GARGANI L, MOTTOLA G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr 2006; 19: 356-363. collapse of inferior vena cava. Am J Cardiol 2005; 66: 493-496. 37) BERNARDI E, CAMPORESE G, BÜLLER HR, SIRAGUSA S, I MBERTI D, B ERCHIO A, G HIRARDUZZI A, V ERLATO F, ANASTASIO R, PRATI C, PICCIOLI A, PESAVENTO R, BOVA C, M ALTEMPI P, Z ANATTA N, C OGO A, C APPELLI R, B UCHERINI E, C UPPINI S, N OVENTA F, P RANDONI P; ERASMUS STUDY GROUP. Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: a randomized controlled trial. JAMA 2008; 300: 1653-1659. 38) BLAIVAS M, FOX C. Outcome in cardiac arrest patients found to have cardiac standstill on bedside emergency department echocardiogram. Acad Emerg Med 2001; 8: 616-621. 39) S ALEN P, M ELNIKER L, C HOOLJIAN C, R OSE JS, A L TEVEER J, REED J, HELLER M. Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients? Am J Emerg Med 2005; 23: 459-462. 40) WANG S, FLEISCHMANN KE. Role of echocardiography in evaluating patients presenting to the emergency room with acute chest pain: Diagnosis, triage treatment decisions, outcomes. In Otto CM (ed): The Practice of Clinical Echocardiography, 2nd ed. Philadelphia, WB Saunders, 2002, pp 235-250. 29) LICHTENSTEIN D, MEZIÈRE G, BIDERMAN P, GEPNER A, BARRÈ O. The comet-tail artifact: An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997; 156: 1640-1646. 41) BROWN JM. Use of echocardiography for hemodynamic monitoring. Crit Care Med 2002; 30: 13611364. 30) LICHTENSTEIN D, MENU Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest 1995; 108: 1345-1348. 42) LANOIX R, LEAK LV, GAETA T, GERNSHEIMER JR. A preliminary evaluation of emergency ultrasound in the setting of an emergency medicine training program. Am J Emerg Med 2000; 18: 41-45. 31) SOLDATI G, TESTA A, PIGNATARO G, PORTALE G, BIASUCCI DG, LEONE A, GENTILONI SILVERI N. The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med Biol 2006; 32: 11571163. 43) TAYAL VS, KLINE JA. Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation 2003; 59: 315-318. 32) LICHTENSTEIN DA. Ultrasound in the management of thoracic disease. Crit Care Med 2007; 35 (5 Suppl): 250-261. 44) NIEMANN T, EGELHOF T, BONGARTZ G. Transthoracic sonography for the detection of pulmonary embolism – A meta-analysis. Ultraschall Med 2009; 30: 150-156. 33) BARBIER C, LOUBIÈRES Y, SCHMIT C, HAYON J, RICÔME JL, JARDIN F, VIEILLARD-BARON A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004; 30: 1740-1746. 34) PINSKY MR. Assessment of indices of preload and volume responsiveness. Curr Opin Crit Care 2005; 11: 235-239. 35) LYON M, BLAIVAS M, BRANNAM L. Sonographic measurements of the inferior vena cava as a marker of blood loss. Am J Em Med 2005; 23: 45-50. 36) KIRCHER BJ, HIMELMANNRB, SCHILLER NB. Noninvasive extimation of right atrial pressure from inspiratory 45) MINIATI M, MONTI S, PRATALI L, DI RICCO G, MARINI C, FORMICHI B, PREDILETTO R, MICHELASSI C, DI LORENZO M, T ONELLI L, P ISTOLESI M. Value of transthoracic echocardiography in the diagnosis of pulmonar y embolism: results of a prospective study in unselected patients. Am J Med 2001; 110: 528-535. 46) JACKSON RE, RUDONI RR, HAUSER AM, PACAUL RG, HUSSEY ME. Prospective evaluation of two-dimensional transthoracic echocardiography in emergency department patients with suspected pulmonary embolism. Acad Emerg Med 2000; 7: 994-998. 87 A. Testa, G.A. Cibinel, G. Portale, P. Forte, R. Giannuzzi, G. Pignataro, N. Gentiloni Silveri 47) LEIBOWITZ D. Role of echocardiography in the diagnosis and treatment of acute pulmonary thromboembolism. J Am Soc Echocardiogr 2001; 14: 921-926. 48) LE GAL G, RIGHINI M, SANCHEZ O, ROY PM, BABAAHMED M, PERRIER A, BOUNAMEAUX H. A positive compression ultrasonography of the lower limb veins is highly predictive of pulmonary embolism on computed tomography in suspected patients. Thromb Haemost 2006; 95: 963-966. 49) K ÜRKCIYAN I, M ERON G, S TERZ F, J ANATA K, D O MANOVITS H, HOLZER M, BERZLANOVICH A, BANKL HC, LAGGNER AN. Pulmonary embolism as cause of cardiac arrest. Arch Intern Med 2000; 160: 15291535. 88 50) COMESS KA, DEROCK FA, RUSSELL ML, TOGNAZZI-EVANS TA, BEACH KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109: 351-356. 51) SUBOTICH D, MANDARICH D. Accidentally created tension pneumothorax in patient with primary spontaneous pneumothorax – confirmation of the experimental studies, putting into question the classical explanation. Med Hypothesis 2005; 64: 170-173. 52) R EARDON R, H EEGAARD B, P LUMMER D, C LINTON J, COOK T, TAYAL V. Ultrasound is a necessary skill for emergency physicians. Acad Emerg Med 2006; 13: 334-336.