* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
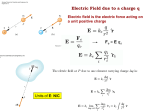
Announcements Physics Department Seminar: TITLE: "The fluid dynamics of climatic variations." SPEAKER: Professor Walter A. Robinson, Department of Atmospheric Sciences University of Illinois at Urbana-Champaign TIME: Thursday Jan. 13, 2005 at 4 PM Essays on Quizzes/Surveys: I do read and comment on them when appropriate. Reserve Books Several books are on reserve in the library: There will be required, supplemental readings from:: Biomedical Applications of Introductory Physics Optional, suggested, readings from:: Electricity and Magnetism, Purcell Div, Grad, Curl and all that For review: First-year Calculus, Hille and Salas Other optional books may be placed for your enjoyment. Matter and Charges •All matter is made of positive and negative charges (or neutral) •An object’s total charge is very close to zero •When an object becomes charged, a tiny fraction of its charged particles (usually electrons) are lost or gained •These particles (usually electrons) can flow through objects •Some materials are better at allowing the flow of electrons than others Conductor A material that allows electrons or other charged particles to flow freely Insulator A material that resists the flow of electrons and other charged particles Elementary Charge •Charges seem to come only in integer multiples of a fundamental charge unit called e •We will treat e as a positive number (some sources treat it as negative) e = 1.602 10-19 C Particle Proton Neutron Electron Oxygen nuc. Calcium ion Chlorine ion q e 0 -e 8e 2e -e know these Some ways to charge objects •By rubbing dissimilar objects •By chemical processes •By proximity between a charge and a conductor – charging by induction •By physical contact between a charge and a conductor + - + Quiz Threeballs pithballs are suspended thin threads. Three are suspended fromfrom thin threads. Various Variousare Objects are thenagainst rubbedother against other Objects then rubbed objects objectsagainst (nylonsilk, against silk, glasspolyester, against etc.) (nylon glass against polyester, and each of the balls is charged and each ofetc.) the balls is charged by touching by touching with objects. one of these objects. It is them with onethem of these It is found that found1 that 1 and 2 repel eachballs other2 and balls and pithballs 2 repel each other and that that3pithballs 2 and 3 repel each other. and repel each other. Fromthis thiswe wecan canconclude concludethat: that: From B) All the1 balls same sign A) Balls and 3carry carrycharges chargesofofthe opposite sign. Charges equalcarry signcharges repel, soof1the andsame 2 have the B) All theofballs sign sameball sign, as dono 2 and 3, and so they all have the C) One carries charge. same sign. to determine without more D) Impossible information. Coulomb’s Law •Like charges repel, unlike charges attract •Force is directly along a line joining the two charges q1 q2 r ke q1q2 Fe 2 r ke = 8.988109 Nm2/C2 •An inverse square law, just like gravity •Can be attractive or repulsive – unlike gravity •Constant is enormous compared to gravity •Obeys the superposition principle just like gravity Coulomb’s Law: Applied A Helium nucleus (charge +2e) is separated from one of its electrons (charge –e) by about 3.00 10-11m. What is the force the nucleus exerts on the electron? Is it attractive or repulsive? ke q1q2 9 Nm2/C2 k = 8.98810 Fe e 2 r r = just 3.00 10-11m the force We calculated the electron q1 =on 3.204 10-19Cfrom the nucleus. How does this q2 =compare -1.602with 10-19the C force on the nucleus from the electron? A) on the nucleus is twice as big Fe=The- force 0.513 N Attractive Force B) The force on the nucleus is half as big C) The forces are equal in magnitude Newton’s Laws and Kinematics Newton’s laws and all the kinematics you learned in 113 are still true! Fnet ma F12 F21 A body in motion tends to stay in motion, therefore changing velocity, i.e. acceleration, requires a force! t' t' dx dv v ;a v adt vt0 ; x vdt x0 dt dt t0 t0 If a does not depend on time, then 1 2 v at v0 ; x at v 0 t x 0 2 Coulomb’s Law vs. Gravity A Helium nucleus (charge +2e) is separated from one of its electrons (charge –e) by about 3.00 10-11m, and we just calculated the electrostatic forces involved. ke q1q2 9 Nm2/C2 k = 8.98810 Fe e 2 r Suppose we could adjust the distance between the nucleus (considered as a point particle) and one electron. Can we find a point at which the electric and gravitational forces are equal? A) Yes, move the particles apart. B) Yes, move the particles together. C) No, they will never be equal. Coulomb’s Law – more versions ke q1q2 Fe rˆ 2 r •Force is a vector (remember from last semester) q1q2 -12 C2/ (N●m2) ˆ Fe r = 8.85410 2 0 4 0 r •Permittivity of free space ke 1 4 0 •Force can be positive or negative. No absolute value signs unless you just want the magnitude! Conductors redistribute charge •Conductor Q Add charges, Q •Conductors allow free flow of charge •Like charges repel •So the charges will redistribute themselves over the sphere Spherical Shells •Just like with gravity, a charge outside feels the charge from the sphere as if it were concentrated in the middle Q q •Consider the forces from opposite charge elements and the vector decomposition Coulomb’s Law My body contains about 31028 electrons, all repelling each other. How come I don’t explode? A) Electrons attract each other, not repel each other B) The gravitational force is so strong it holds me together, overcoming electric forces C) There are also positive charges that cancel out the negative charges D) Electrical forces are too weak to consider Electric Fields •Electric Field is the ability to extert a force at a distance on a charge •It is defined as force on a test charge divided by the charge •Denoted by the letter E •Units N/C + + – –– + + + F Small test charge q E F /q E-Field: Why? •When we have a charge distribution, and we want to know what effect they would have external charges, we can either •Do many sums (or integrations) every time a charge comes in to find the force on that charge •Or calculate the field from the charge distribution, and multiply the field by the external charge to obtain the force •Simplification! Electric Field from a Point Charge keQq F 2 rˆ r E F /q ke Q E 2 rˆ r Point charge Q Small test charge q Note: the field is a vector! Quiz Two test charges are brought separately into the vicinity of a charge +Q. First, test charge +q is brought to point A a distance r from charge +Q. Next, the +q charge is removed and a test charge +2q is brought to point B a distance 2r from charge +Q. Compared with the electric field of the charge at A, the electric field of the charge at B is: • T +Q +q +Q A +2q B A) Greater B) Smaller C) The same.