* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Polysubstance dependence wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Compounding wikipedia , lookup
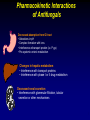
Orphan drug wikipedia , lookup
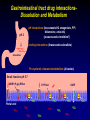
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
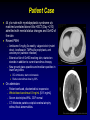
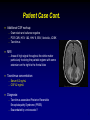
Managing Drug Interactions in the Patient with Aspergillosis Russell E. Lewis, Pharm.D., FCCP Associate Professor University of Houston College of Pharmacy/ The University of Texas M.D. Anderson Cancer Center 1 Patient Case 44 y/o male with myelodysplastic syndrome s/p matched unrelated donor Allo-HSCT (Day +210) admitted with mental status changes and GvHD of the skin Recent PMH: Ambisome 5 mg/kg 3x weekly, valganciclovir (maint dose), levofloxacin, TMP/sulfa prophylaxis, and vancomycin (catheter infection) Extensive flair of GvHD involving skin, started on steroids in addition to current tacrolimus therapy New ground glass opacities and nodular opacities in lower lung lobes DC Ambisome, start voriconazole Reduce tacrolimus dose by 30% On admission: Patient confused, disoriented but responsive Whole blood tacrolimus 6.9 ng/mL [5-15 ng/mL] Serum electrolytes WNL, CSF normal CT: Moderate parieto-occipital cerebral atrophy without focal abnormalities. 2 Patient Case Cont. Additional CSF workup: Gram stain and cultures negative PCR CMV, HSV 1&2, HHV 6, EBV, Varicella, JC/BK Tacrolimus MRI Areas of high signal throughout the white matter particularly involving the parietal regions with some extension on the right to the frontal lobe Tacrolimus concentration: Serum 6.2 ng/mL CSF 42 ng/mL! Diagnosis: Tacrolimus associated Posterior Reversible Encephalopathy Syndrome (PRES) Exacerbated by voriconazole? 3 Factors that Increase the Potential for Serious Drug Interactions with Antifungal Therapy Polypharmacy Underlying renal or hepatic dysfunction Drugs with narrow therapeutic index Debilitation /malnutrition/ chronic immunosuppression Genetic predisposition (I.e. poor metabolizer) Risk is cumulative, and the relative impact each factor at different timepoints in unknown 4 Classification of Drug Interactions Pharmacokinetic ∆ in drug absorption, distribution, metabolism or excretion Pharmacodynamic ∆ of pharmacological effect at standard drug concentrations or ∆ of pharmacological effect resulting from altered pharmacokinetic exposures “All drugs known to humans are poisons, only the amount or dose determine the effects.” Paracelsus, 1490 - 1541 5 Pharmacodynamic Interactions of Antifungals Beneficial: Synergy (e.g., echinocandin + triazole) Suppression of resistance (e.g., 5-FC + amphotericin B) Detrimental: Antagonism (e.g., triazole + amphotericin B) Overlapping toxicities Amphotericin B + other nephrotoxic drugs Amphotericin B nephrotoxicity accumulation of renally-eliminated drugs electrolyte disturbances diuretics enhanced toxicity of steroids digoxin, skeletal muscle relaxants Azoles + steroids adrenal suppression All antifungals hepatic toxicity 6 Pharmacokinetic Interactions of Antifungals Decreased absorption from GI tract • Alterations in pH • Complex formation with ions • Interference w/transport protein (i.e. P-gp) • Pre-systemic enteric metabolism Changes in hepatic metabolism • Interference with transport proteins • Interference with phase I or II drug metabolism Decreased renal excretion • Interference with glomerular filtration, tubular secretion or other mechanisms 7 Azoles are susceptible to pharmacokinetic interactions in the GI tract Dissolution Aqueous solubility N N N N CH3 F O O H3C N N N N N N N N N O O N N OH Fluconazole pKa 2 F N N N F H N OH F Cl N N H3C CH3 N F Voriconazole pKa 1.63 Cl Itraconazole pKa 3.7 log P-5.66 O O H3C N HO N N N O N H F F Posaconazole pKa 3.6 log P-3 Lipid solubility 8 Gastrointestinal tract drug interactionsDissolution and Metabolism pH 2 pH interactions (itraconazole-H2 antagonists, PPI, didanosine, antacids) (posaconazole-cimetidine?) binding interactions (itraconazole-sulcralfate) dissolution Pre-systemic clearance/metabolism (all azoles) Small intestine pH 5-7 MDR1 (P-gp) Efflux CYP 3A4 OATP Portal vein 9 Hepatic Drug Interactions Genetic Disease OATP (azoles, echinocandins?) Diet Drugs Infection Phase I metabolism (CYP P450) (itraconazole, voriconazole) Phase II metabolism (glucoronidation) (posaconazole) Extraction? Metabolism 10 All azoles are inhibitors of CYP Affinities for specific CYP isoforms are drug dependent 11 In Vivo Cytochrome P450 Inhibition Potential vs Other Azoles CYP3A4 Drug Substrate Inhibitor Itraconazole2,3,4 Ketoconazole2,3,5 Voriconazole3,6,7 Posaconazole1 Fluconazole2,3 1. 2. 3. 4. 5. 6. 7. Inhibitor CYP2C8/9 Wexler D et al. Eur J Pharm Sci. 2004;21:645-653. Cupp MJ et al. Am Fam Phys. 1998;57:107-116. Drug interactions. Med Letter. 2003;45(W1158B):46-48. Sporanox IV [summary of product characteristics]. Bucks, UK; Janssen-Cilag Ltd; 2005. Nizoral tablets [summary of product characteristics]. Bucks, UK; Janssen-Cilag Ltd; 2001. Hyland R et al. Drug Metab Dispos. 2003;31:540-547. VFEND [summary of product characteristics]. Kent, UK; Pfizer Ltd; 2005. Substrate CYP2C19 Inhibitor Substrate 12 Itraconazole 3A4 Interactions Affecting Pharmacokinetics of Other Drugs Drug Effect Alternatives/Management HMG-CoA reductase (lovastatin, simvastatin, atorvastatin) 3-20 fold Cmax, AUC0-24, t1/2 Fluvastatin, pravastatin, rosuvastin Cmax, AUC, t1/2, F, clearance Oxazepam, estolazam, temazepam Anxiolytics, sedatives (buspirone) 13-fold Cmax, AUC0-24 Zolpidem Antipsychotics (Haloperidol) 30% AUC Clozapine Immunosuppressants CsA Tacrolimus Cmin >50% Cmin 5-fold Empirically reduce dosage by 50% and monitor levels Corticosteroids Methylprednisolone, dexamethazone Prednisolone 3-4x increase in AUC 15-30% increase in t1/2 Adrenal-suppressant effects Calcium channel blockers Felodipine 6-8x fold increase in AUC Avoid Chemotherapy (Cyclophosphamide, busulfan, vinca alkaloids) Css > 25-50% Avoid concomitant use, especially for conditioning therapy Benzodiazepines (midazolam, triazolam, diazepam) 13 Cyclophosphamide metabolism is affected by azole antifungals Urine DCCY fluconazole Fluconazole CY HCY Itraconazole ketoCY HPMM CEPM CYP 2B6 2C9, 2C19 3A4 Itraconazole aldoCY acrolein Cyclophosphamide metabolism changes at different dosages (Timmet al Pharmcogenom J 2005;5:365) Marr et al. Blood 2004;103:1557 14 Itraconazole 3A4 Interactions and Anti-Mycobacterial or HIV Drugs Drug NNRTI (delavirdine, nevirapine, efavirenz) Protease inhibitors (Indinavir, aprenavir, saquinavir) (lopinavir, ritonavir) Rifabutin Effect Alternatives/Management Decreased metabolism of NNRTIs, Nevirapine and efavirenz may induce itraconazole metabolism Monitor for antiviral toxicity and antifungal efficacy/ itraconazole trough concentrations Increased PI concentrations Increased ITRA concentrations Indinavir 600 mg q8h Monitor for toxicity Rifabutin induces metabolism of itraconazole, itraconazole inhibits metabolism of rifabutin Rifabutin uveitis, antifungal efficacy/ itraconazole trough concentrations 15 Voriconazole Interactions Affecting Pharmacokinetics/Dynamics of Other Drugs Drug (Enzyme) Effect Management Inhibits primary metabolic pathway, increases PD effect by 41% Monitor INR and adjust dose accordingly Immunosuppressants (3A4) • Cyclosporin • Tacrolimus • Sirolimus Cmin 248%, AUC 70% Cmin Cmin Reduce dose by 50%, monitor Reduce dose by 33%, monitor Contraindicated Miscellaneous (2C9, 3A4) • Phenytoin • Omeprazole • Prednisolone • Rifabutin Cmax 70%, AUC 80% Cmax 2.5 fold, AUC 3.8 fold AUC 13-30% AUC, 2-fold Monitor phenytoin levels Reduce dose by 50% Monitor Warfarin (CYP 2C9) Voriconazole may also increase the plasma concentrations of several drugs including benzodizepines, calcium channel blockers, HMG-CoA reductase inhibitors, vinca alkaloids, busulfan, cyclophosphamide sulfonylureas, protease inhibitors, NNRTI’s, sirolimus, quinidine and pimozidine, however, published studies are lacking. 16 Posaconazole Interactions Affecting Pharmacokinetics/Dynamics of Other Drugs Drug Effect Management Immunosuppressants (3A4) • Cyclosporine • Tacrolimus Cmin 14-24% AUC 360% Monitor Reduce dose by 50%, monitor Miscellaneous (3A4) • Phenytoin • Rifabutin • Ritonavir AUC 15%, Posa 50% AUC 82%, Posa 50% AUC 30% Monitor phenytoin levels Avoid if possible, monitor for uveitis Clinically significant? Posaconazole may also increase the plasma concentrations of several drugs including benzodizepines, calcium channel blockers, HMG-CoA reductase inhibitors, vinca alkaloids, busulfan, cyclophosphamide, sulfonylureas, protease inhibitors, NNRTI’s, sirolimus, quinidine and pimozidine, however, published studies are lacking. 17 Summary-Important CYP-Azole Interactions Drug Interaction Azole + Cytochrome P450 Inducers Carbamazepine Phenobarbitol Phenytoin Isoniazid Azole concentration Rifabutin Rifampin Nevirapine Azole + Cytochrome P450 Substrate Statins Cyclosporine Tacrolimus Sirolimus Substrate concentration Protease inhibitors (saquinavir, ritonavir) Ca2+ channel blockers (diltiazem, verapamil, nifedipine, nisoldipine) 18 Antifungal Serum Drug Concentration Monitoring Agent Justified in select situations? No Yes- toxicity Target Range Fluconazole Itraconazole No Yes-ensure absorption, efficacy N/A > 0.5 mcg/mL Voriconazole Yes-variable metabolism associated with sub-therapeutic and to xic concentr ations drug interact ions, pediatrics? Yes, ensure absorption, efficacy 1-2 to 6 mcg/mL No N/A Amphotericin B* Flucytosine Posaconazole Echinocandins * Including lipid preparations N/A < 100 mcg/mL > 0.25 mcg/mL? Timing of Sample N/A 2 hour postdose peak N/A Trough after 7 days of therapy Trough after 7 days of therapy Trough after 7 days of therapy N/A 19 Distribution of Poor Metabolizers of CYP P450 2C19 in Various Ethnic Groups Influence of CYP2C19 Genotype on Average Steady-State Plasma Voriconazole Concentrations Genotype Caucasian Blacks Japanese Chinese Homozygous poor metabolizer 2% 2% 19% 14% Heterozygous extensive metabolizer 26% 28% 46% 43% Homozygous extensive metabolizer 73% 70% 35% 43% Homozygous Extensive metabolizer (n=108) Clin Pharmacokinet 2002;41:913-958. Heterozygous Extensive metabolizer (n=39) Homozygous Poor metabolizer (n=8) 20 Pharmacogenomic microarray typingCleared in U.S. and EU for Diagnostic Use CYP450 Array The world's first pharmacogenomic microarray designed for clinical applications that provides comprehensive coverage of gene variations – including deletions and duplications – for the 2D6 and 2C19 genes, which play a role in the metabolism of about 25% of all prescription drugs. It is intended to be an aid for physicians in individualizing treatment doses for patients on therapeutics metabolized through these genes. Cost- ~ $500/ test 21 Antimicrobials and QTc ProlongationRelative Risk for Torsades de Pointes (TdP) Schedule I: Highest TdP risk, potent Ikr blockers, TdP risk > 1% Dofetilide Sotalol Cisapride Terbinafine Schedule II: Significant risk for TdP, particularly when co-administered with CYP inhibitors Clarithromycin Erythromycin (IV>PO) Sparfloxacin Itraconazole Ketoconazole Pentamidine Schedule III: Significant risk for TdP, particularly when co-administered with CYP inhibitors Schedule IV: Low risk for TdP, case reports of TdP, mild Ikr blockade, possible CYP interactions Schedule V: Questionable/minimal risk for TdP Gatifloxacin Levofloxacin Moxifloxacin Grepafloxacin Azithromycin Gemifloxacin* Fluconazole Voriconazole* Telithromycin* Cotrimoxazole Ciprofloxacin *New antimicrobials, based on post-marketing data may be re-categorized 22 RC Owens Drugs 2004;64:(10):1091-1124. H2N NH OH O HO O NH H H2N N H O N O OH HN H3C H H NH HO H O CH3 CH3 CH3 O H N H OH OH O OH caspofungin HO OH O HO HO O NH H3C HO NH H2N N O O HN H3C NH O O HO NH O O NH N OH OH O HO N CH3 O O H3C HN H N HO HO NH O OH OH O OH O H N HO O CH3 N O S OH N O H3C O O OH O OH HO micafungin O anidulafungin HO H3C 23 Comparison of the Echinocandin AntifungalsSafety Caspofungin Micafungin Anidulafungin CYP 3A4 inhibitor? No No No Drug interactions OATP1B1 transporter? Tacrolimus 20% CSA CASPO 35% RIF or other inducers CASPO 30% No effects on tacrolimus,cyclosporine, prednisolone or effects of rifampin. Sirolimus, nifedipine AUC 20% No effects on tacrolimus,cyclosporine, prednisolone or effects of rifampin. Dosage adjustment in hepatic dysf. To 35 mg/day in moderate hepatic insufficiency No dosage adjustment No dosage adjustment Adverse effects Histamine-rxn with infusion, phlebitis, Asymptomatic transminases Occasional histaminerxn with infusion, phlebitis, Asymptomatic transaminases N&V, headache, hypokalemia, and GGT Summary • Patients with invasive aspergillosis have many risk factors for potentially harmful drug interactions, some of which may be unanticipated • A pro-active approach is essential to protect patients from potentially severe interactions – Better laboratory support may help the management of suspected interactions (serum drug level monitoring, genotyping?) • Drug interactions that are always significant: – Interactions affecting agents with narrow therapeutic index (e.g., immunosuppressants, chemotherapy, anti-retrovirals) – Interactions increasing the metabolism of antifungals used to treat the Aspergillus infection – Interactions affecting the QTc (Torsades de pointes) "The person who takes medicine must recover twice, once from the disease and once from the medicine." - William Osler, M.D. 26