* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The global burden of Congenital Rubella Syndrome
Survey
Document related concepts
Meningococcal disease wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Oesophagostomum wikipedia , lookup
Cysticercosis wikipedia , lookup
Neonatal infection wikipedia , lookup
Poliomyelitis eradication wikipedia , lookup
Trichinosis wikipedia , lookup
Hepatitis B wikipedia , lookup
Anthrax vaccine adsorbed wikipedia , lookup
Neisseria meningitidis wikipedia , lookup
Whooping cough wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Transcript
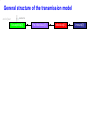
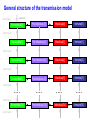
The global burden of Congenital Rubella Syndrome Emilia Vynnycky, Elisabeth Adams and many others Health Protection Agency Centre for Infections London, UK Background • Previous review of the burden of rubella and Congenital Rubella Syndrome (CRS) in developing countries was conducted for 1996 (Cutts and Vynnycky) • Total numbers of CRS cases in 1996: 110,000 (95% range: 14,000-308,000) • Number of countries outside Europe which had not introduced rubella-containing vaccine: 1996: 126 (~2/3 developing countries) 2008: 67 Overview of the study Aims • To estimate the annual burden of CRS during 2000-2008, for the 193 WHO member states, six WHO regions and globally • To revise previous estimates for 1996 to include countries which had already included rubella vaccination Literature search 16 databases searched; studies excluded if they were conducted among biased populations e.g. Health-care workers, refugees, samples collected among individuals suspected to have rubella Modelling methods Depended on whether the setting had introduced rubella vaccine by 2000 Examples of the variable quality of available datasets % seronegative 100 Ethiopia (females), 1994 (Cutts et al, 2000) 100 75 75 50 50 25 25 0 0 0 10 20 30 40 50 Brazil - Mato Groso du Sul, 2002-3 (Figuero-Filho et al, 2007) 100 75 0 100 50 25 25 0 0 10 20 30 10 20 30 40 50 40 50 Niger, ? (Develoux et al , 1991) 75 50 0 Nigeria ,? (Bukbuk et al, 2002) 40 50 0 Age (yrs) 10 20 30 Data for populations in which rubella-containing vaccine had not been introduced by 2000 • 31 datasets available on the age-specific proportion of individuals seronegative for rubella • 20 countries covered by the data: • 14 African countries • 8 datasets from India, 2 from Pakistan and Yemen Number of studies (NB: ~67 countries had not yet introduced rubella vaccine by 9 2000) 8 7 6 5 4 3 2 1 0 Year of study Methods for populations in which rubellacontaining vaccine had not been introduced • A catalytic model was fitted to the data to estimate the force of infection (rate at which susceptible individuals are infected) among children and adults Predictions from a simple catalytic model: General structure of a Immune 100 catalytic model: Susceptible Immune % 50 Susceptible 0 Age • Where possible, the sensitivity of the antibody test was also estimated Examples of typical datasets 100 100 Congo, ? (Yala at al, 1991) % susceptible (=seronegative) 75 75 50 50 25 25 0 0 0 10 100 20 30 40 Pakistan, ? (Iqbal and Bokhari, 1997) 0 50 India (Lucknow), 1972-3 (in Seth, 1985) 75 50 50 25 25 20 30 40 50 Kenya, 1996-9 (Shulman, unpublished) 100 75 0 10 0 0 10 20 30 40 50 Age (years) 0 10 20 30 40 50 Force of infection estimates for those aged ≥13 years for the available datasets in countries in which rubellacontaining vaccine had not been introduced by 2000 Methods for unvaccinated populations – equations for the CRS incidence • CRS incidence per 100,000 live births in a given age range = Proportion susceptible in that age range Risk that a child is × Risk of infection during 16 weeks born with CRS =65% × 0.65 × 100,000 if infection occurs during the first 3 months of pregnancy • If multiple data sources were available, CRS incidence taken as the average obtained for each data set • If no serological data: CRS incidence taken as the regional average • Number of new CRS cases each year among pregnant women in a given age range = Age-specific CRS incidence per live birth × numbers of births Estimates of the incidence of CRS in populations in which rubella-containing vaccine had not been introduced by 2000 Limitations of estimates for populations in which rubella-containing vaccine has not been introduced • Only 33% of serological studies are from the 1990s/2000; year of study is unknown for 33% of available datasets • Data are available from only 20 out of the 67 countries that had not introduced rubella-containing vaccine • Sampling methods are sometimes unclear • Data quality is sometimes very poor e.g. few datapoints, large confidence intervals, test quality is unclear •etc Methods for populations in which rubella-containing vaccine has been introduced by 2000-2007 1. Use a model to describe the transmission of rubella among males and females in the given setting before the introduction of vaccination 2. Incorporate reported vaccine coverage over time in the model General structure of the transmission model end of year newborns Susceptible[0] Pre-Infectious [0] Infectious[0] Immune[0] General structure of the transmission model newborns end of year Susceptible[0] Pre-Infectious [0] Infectious[0] Immune[0] Susceptible[1] Pre-Infectious[1] Infectious[1] Immune[1] Susceptible[2] Pre-Infectious [2] Infectious[2] Immune[2] Susceptible[3] Pre-Infectious [3] Infectious[3] Immune[3] …. …. end of year end of year end of year end of year …. …. end of year Susceptible[74] end of year Pre-Infectious [74] Infectious[74] Immune[74] Force of infection (%/year) An example of the predicted force of rubella infection over time in England for 2000-2008, using the transmission model Year Methods for populations in which rubella-containing vaccine has been introduced by 2000-2007 1. Use a model to describe the transmission of rubella among males and females in the given setting before the introduction of vaccination 2. Incorporate reported vaccine coverage over time in the model 3. Calculate the CRS incidence per 100,000 livebirths in a given age range for each year, using the formula: = Proportion susceptible in a given age range × Risk of infection during 16 weeks × Use observed proportion 0.65 × 100,000 seronegative if available, or model predictions Number of CRS cases per 100,000 live births Predictions of the incidence of CRS in 2000 and 2008 in Europe Country Average incidence of CRS per 100,000 live births, 2008 Average CRS incidence per 100,000 live births <50 50-<100 100-<150 >150 No data Source: Vynnycky, Adams et al (in preparation) Average number of CRS cases born in 2008 Number of CRS cases <10 10-<100 100-<1000 1000-<10000 10,000 or more No data Source: Vynnycky, Adams et al (in preparation) Estimates of the global burden of CRS, 2000-2008 300000 2000 Number of CRS cases 250000 200000 150000 100000 50000 0 Region 2008 Estimates of the global burden of CRS, 1996-2008 300000 1996 2000 Number of CRS cases 250000 200000 150000 100000 50000 0 Region 2008 Conclusions • Average global burden of CRS has remained relatively unchanged since 2000 with 112,000 cases per year; very wide range: 16,000300,000 • Average global burden has decreased slightly since 1996 (~120,000 cases born worldwide, in 1996; 110,000 in countries which had not yet introduced rubella vaccine) Conclusions • The average burden may have increased in African, SE Asian countries due increased numbers of births over time • Burden remains the highest in countries which have not yet introduced vaccination • The burden has declined in countries which did introduce adequate vaccination • Further adequate serological studies are needed to improve the reliability of the estimates Acknowledgments HPA Elisabeth Adams*** Nigel Gay Ben Cooper Richard Pebody Andrew Vyse Tony Nardone David Brown Albert Jan van Hoek Other Felicity Cutts (LSHTM) James Nokes (KEMRI) Dani Cohen (Israel) Stephanie Meredith (WHO) Susan Reef (CDC) Karen Shulmann Emily Simons (WHO) Peter Strebel (WHO) Alya Dabbagh (WHO) Ann Navarr (JHSPH) Neal Halsey (JHSPH)