* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Engineering Chemistry (revised edition)
History of metamaterials wikipedia , lookup
Molecular nanotechnology wikipedia , lookup
History of nanotechnology wikipedia , lookup
Impact of nanotechnology wikipedia , lookup
Industrial applications of nanotechnology wikipedia , lookup
Nanotechnology wikipedia , lookup
Nanomedicine wikipedia , lookup
Colloidal crystal wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
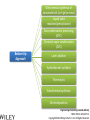
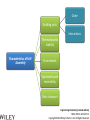
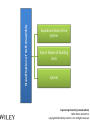
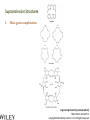
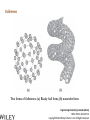
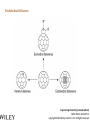
Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Introduction Nanotechnology can be defined as the manipulation of atoms and molecules at nano (one billionth) scale (1–100 nm) to produce devices, structures or systems having at least one novel or superior property. The materials having at least one dimension in the nano scale are called nanomaterials. Properties of Nanomaterials 1. The surface area to volume ratio of the nanomaterials is relatively larger than that of bulk materials of the same mass. This increases the chemical reactivity and affects strength and electrical properties of the material. 2. The quantum confinement is observed at nanometer sizes that changes the optical, electronic and magnetic properties of the material. The band gap increases as the size of the material is reduced to nanometer range. This effect is caused by the phenomenon resulting from electrons and electron holes being constricted into a dimension which approaches the critical quantum measurement, known as the exciton Bohr radius. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved The combination of number of possible materials coupled with the concepts of nanochemistry is mind boggling. A few of them are: 1. Nanoparticles of various sizes and shapes such as, gold, silver nanoparticles. 2. Nanooxides such as iron oxide, cadmium oxide 3. Carbon nanotubes and fullerenes 4. Nanopolymers and nanomembranes 5. Nanocatalysts 6. Nanoformulations for cosmetics, example, in sunscreens, anti-aging creams, etc. 7. Nanoceramics These materials have, in turn, applications in various domains: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. In semiconductor devices, such as nanocapacitors As therapeutic molecules (nanomedicine) for diseases such as cancer. In targeted drug delivery In agriculture In electronics As taste enhancers (nanofoods) and for packaging in food industry In fuel cells In chemical and biosensors In fabric technology In solar cells Nanooptics For manufacture of self-cleaning surfaces In waste water treatment Green nanotechnology Size-Dependent Properties Particle-Size vs. Surface Area If a macroscopic object is divided into smaller parts, the ratio of surface atoms to interior atoms becomes a significant number of the total fraction of atoms. Surface Chemistry of Materials Due to increased surface area, nanomaterials possess greater surface energy and are thermodynamically less stable. The atoms or molecules that exist on the surface or the interface are somewhat different from atoms/molecules in the bulk/interior of the material. Electronic Properties Various physical properties such as thermal, optical and magnetic properties are dependent on the electronic state of the material. Some characteristics of electronic structure of solids are: 1. In an isolated solid, the electrons move in quantified energy levels. When the distance between atoms is less, electron orbitals interact with each other, which leads to broadening of energy levels to form energy bands. 2. The inner-shell electrons form narrow bands called internal bands and electrons in external shell form valence bands. The electrons in excited state form conduction band. 3. The difference of energy between valence band and conduction band is called energy gap. In metals (or conductors) this energy gap is zero; it is small in case of semiconductors and large in case of insulators. 4. The maximum energy for electrons at absolute zero (0 K) is called Fermi level or Fermi energy. The physical properties of materials are mainly governed by electrons that have energy larger than Fermi energy. Mechanical Properties Nanocrystalline materials have an average crystallite size in the range 1 to 100 nm and are characterized by numerous grain boundaries due to the small size of the grain. The mechanical properties of nanocrystalline materials are determined by their small grain size and the grain boundary. These properties are enhanced by reducing the grain size, as grains of nano-size have no defects inside, unlike micro-grains of relatively larger size. For example, nanocrystalline copper is found to be three times more resistant to applied stress than normal copper crystals and deformed homogeneously. The crystal-linity of the grain structure is maintained right up to the grain boundary. The fraction of atoms out of the total volume present at the grain boundaries is large for small size grains and decreases with increase in their size. General Methods of Synthesis Schematic representation of the building up of nanostructures. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Wet chemical synthesis of nanomaterials (sol–gel process) Liquid solid reactions(precipitation) Gas condensation processing (GPC) Chemical vapor condensation (CVC) Bottom-Up Approach Laser ablation Hydrothermal synthesis Thermolysis Solvothermal synthesis Electrodeposition Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Top-Down Approach High-energy ball milling is the milling process commonly used in ceramic processing and powder metallurgy, and involves particle size reduction, solid-state alloying, mixing or blending, and particle shape changes. The process is used only for hard, brittle materials which fracture, deform and cold weld during the milling operation. The technique has been extended to produce a variety of nanocrystalline, amorphous and quasicrystalline materials. Some disadvantages associated with the process are: 1. All particles of the precursor may not break down tothe required particle size. 2. The nanocrystalline materials prepared by this process may be contaminated by milling tools and atmosphere. This can be reduced by minimizing the milling time, using pure and ductile metal powders which can coat the milling tools and seal the container to prevent exposure to atmospheric gases. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Characterization of Nanomaterials Characterization of nanoparticles is important to understand and control their synthesis and applications. However, the main challenge faced currently in characterization of these materials is the inability and lack of instruments to observe, measure and manipulate the materials at the nanoscale. Common techniques are electron microscopy (TEM, SEM), atomic force microscopy (AFM), dynamic light scattering (DLS), X-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF), ultraviolet–visible spectroscopy, dual polarization interferometry and nuclear magnetic resonance (NMR). Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Materials Self-Assembly Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Order Building units Interactions Thermodynamic stability Characteristics of SelfAssembly Environment Spontaneity and reversibility Mass transport Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Classification of Self-Assembly Equilibrium State of the System Size or Nature of Building Units System Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Classification of self-assembly based on size or nature of the building units Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Formation of Self-Assembly Self-Assembled Monolayers (SAMs) Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Electrostatic Self-Assembly (ESA) or Layer-by-Layer (LbL) Self-Assembly Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved There are several advantages in this method over other thin film deposition techniques: 1. The thin films formed by this technique are highly stable. 2. LbL method offers high level of control over the thickness of the film because of the linear growth of the layers. 3. This method is simple, inexpensive and can be automated Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Mechanism of Self-Assembly In terms of forces acting in the process, self-assembly occurs when a balance is established between three different classes of forces, namely, attractive (driving) forces, repulsive (opposition) forces and directional forces. Formation of micelles or micellization from surfactant molecules is a classic example of self assembly Micelles are surfactant aggregates formed when surfactant molecules are dispersed in aqueous solution at a particular concentration known as the critical micelle concentration (CMC). Directed Self-Assembly Directed self-assembly is quite distinct from spontaneous self-assembly. Physical, chemical and geometrical cues can be added to a self-assembly process so that a desired structure may be obtained. Directing a self-assembly may involve lithographically patterning a substrate on which self-assembly occurs, so that selfassembly occurs only on specific regions. Honeycomb structured patterns, grid structures, trenches and so on, have been formed by self-assembly directed by lithographic patterning. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Molecular Self-Assembly Spontaneous assembly of molecules, without any external intervention is said to be molecular selfassembly. Formation of molecular crystals, phase separated polymers, colloids, lipid bilayers, protein folding, nucleic acid folding, selfassembled monolayers (SAMs) all are examples of molecular selfassembly. There are two types of molecular self-assembly: 1. Intermolecular self-assembly 2. Intramolecular self-assembly. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Molecular vs. Materials Self-Assembly The principles and characteristics of molecular self-assembly are the same as selfassembly of materials. Molecular self-assembly also proceeds from a less ordered state to a highly ordered structure. But there is a difference in the forces directing the process. The self-assembly of materials is directed by a wide range of forces based on the nature of the building units, such as non-covalent weak interactions, elastic, colloidal, capillary, gravitational forces, etc. On the other hand, molecular self-assembly is restricted to the weak non-covalent interactions such as hydrogen bonding, metal coordination, van der Waals forces and π - π interactions. The structure formed by molecular self-assembly is determined by the molecular structures. A variety of structures of different sizes and shapes can be synthesized by molecular self-assembly. It is feasible to construct challenging molecular topologies using molecular self-assembly. For example, molecular analog of Borromean rings has been constructed using DNA. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Two-Dimensional Assemblies 1. Interfaces (region where there is a gradual change of physicochemical properties from one phase to the adjacent phase) can undergo direct selfassembly along certain directions by providing physical, geometrical and chemical spaces. 2. This type of self-assembly is known as interfacial or surface self-assembly. Since there is a restriction of at least one of the dimensions of self-assembly in the length scales of the components, it is also known as two-dimensional self-assembly. 3. The average size of this assembly is 10 nm. It differs from one-dimensional assemblies as one dimensional assemblies form arrays while two-dimensional assemblies form lattice. 4. It is different from three-dimensional or bulk self-assembly because the interactions that take place between the interface are inevitable. The strength of such interactions may vary, but is usually comparable to colloidal and intermolecular forces between the building units. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved This intrinsic factor furnishes three typical, unique characteristics to twodimensional self-assembly that differentiate it from three-dimensional selfassembly: 1. For bulk self-assembly, it is a prerequisite that the building units are amphiphilic, so that proper balance of forces is ensured. But in the case of interfacial self-assembly, the building units may or may not be amphiphilic. 2. The intermolecular and colloidal forces among the building units are altered by their interaction with the interface. For example, on adsorption onto interfaces, even non-polar molecules can acquire dipole moments. This characteristic provides additional means of control over the self-assembly processes at interfaces. 3. The self-assembled aggregates formed in interfacial self-assembly are found to have significantly different chemical and physical properties than those in bulk self-assembly. This is because the self-assembled aggregates in interfacial self-assembly prevail in a state of confinement on the interfaces. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Interfaces are formed spontaneously between any two phases. Interfaces have zero thickness geometrically and macroscopically and therefore are twodimensional. However, microscopically and physicochemically, interfaces are three-dimensional, having a thickness in the range of approximately 1 nm-1 μm (Figure). Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Forces Involved in Interfacial Self-Assembly The interfaces, having thickness in the nanometer range, act as nanometer scale surface wells when the building units are adsorbed onto them. This is because the interplay of intermolecular forces of the building units is confined to the interfaces. As the physicochemical properties change gradually within the interface, the interaction between the building units becomes more favorable along the interface direction and less favorable through either phase direction (either phase 1 or 2). The intermolecular and colloidal forces between the building units can act as either attractive or repulsive forces for the process of interfacial self-assembly. But the direction of the interfacial self-assembly is determined by the interaction between the building units and interfaces and is thus referred to as ‘intrinsic directional force’. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved General Strategy of Two-Dimensional Self-Assembly The general strategy of a two-dimensional self-assembly can be explained as follows: 1. The self-assembly process is initiated when the building units are first located at the interface. This is a prerequisite condition, regardless of the type of the interface. This is usually achieved by adsorption, mainly physisorption. 2. When the force balance between the building units is fulfilled, the building units at the interface self-assemble form self-assembled aggregates. 3. The force balance determines the mode of packing of the building units, the specific structure of the self-assembled aggregates and the prospect of subsequent higher order self-assembly. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Examples of Two-Dimensional Self-Assembly 1. Self-Assembly at the Gas-Liquid Interface 2. Self-Assembly at the Liquid-Solid Interface 3. Self-Assembly at the Liquid-Liquid Interface 4. Self-Assembly at the Gas-Solid Interface Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Formation of Langmuir monolayer Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Mesoscale Self-Assembly (MESA) 1. Mesoscale self-assembly (MESA) is the self-assembly of objects ranging in size from 10 nm to 10 mm through non-covalent forces such as capillary, magnetic, electrostatic and light forces. This involves the extension of ideas emerging from molecular self-assembly to larger objects. 2. Regular arrays of topologically complex, mesoscale objects can be prepared by self-assembly in fluids, with the structure of the arrays determined by shapes of the assembling objects, the wettability of their surfaces and the lateral capillary forces at the interfaces. 3. Self-assembly results from minimization of the interfacial free energy of the liquid-liquid interface. The capillary interactions between objects can be viewed as a type of ‘bond’ that is analogous to chemical bonds that act between atoms and molecules. 4. Assembly by capillary forces relies upon particle-particle interaction and interaction between particles and their environment. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Coercing Colloids Coercing colloids are 3D photonic crystals of colloidal particles with periodic dielectric lattices an optical analog of electronic semiconductor (periodicity of potential in semiconductor crystal affecting the motion of electrons). The size of these colloidal particles is 1-100 nm. These colloidal crystals and films are prepared by electrophoresis, sedimentation, evaporation induced self-assembly, application of electric or magnetic field. Applications of Coercing Colloids They find applications in signal processing, switching and sensing of light, optical computing and telecommunications. Three-dimensional colloidal photonic crystal can be utilized as optical pulse control devices for ultra-short laser pulses (femto second) and high power laser application. Bioinspired colloidal photonic crystals also find application in catalysis, chromatographic separations and color-based sensors for monitoring changes in environment. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Supramolecular Structures 1. Host-guest complexation Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved 2. Self-assembly between complementary molecules 3. Crystal lattice self-assembly: By specific selection of the functional molecular building blocks, the weak and non-covalent bonds lead to the formation of crystal packing In this case, no difference in size is observed and no species acts as a host to the other. Self-assembly and selforganization leads to the formation of organized, discrete aggregates of various intriguing structures like boxes, squares, helicates, catenanes, capsules, grids and many others. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Applications of Supramolecular Structures 1. Self-organized supramolecular organic nanostructures have potential applications in molecular electronics, photonics and as precursors for nanoporous catalysts. 2. Supramolecular metal-ligand assembly finds application as host for catalytic reactions in organic chemistry. 3. Supramolecular crystal lattice self-assembly opens up new avenues for novel nanostructured materials with unique functional properties and for miniaturization and nanofabrication technologies. 4. Supramolecular architectures are used to obtain efficient near infrared photoluminescence and electroluminescence using a three-dimensional p conjugation. 5. SAMs are highly ordered supramolecular arrays of molecules chemisorbed on metal substrates. 6. They allow reactions to take place in the solid state. This leads to reduction in use of solvents and they can also be used in green chemistry.. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Nanoscale Materials Nanocrystals and Clusters Applications of Nanocrystals and Clusters 1. Many techniques are developed to produce clusters for use in different applications like thin film manufacture for advanced electronic or optical devices, production of nanoporous structures, and fabrication of thin membranes of nanoporous materials. 2. Nanoclusters of technologically important inorganic bulk materials such as SiO2, ZnO, CdS, etc., were found to be photoinitiators while their corresponding bulk materials did not exhibit this property. ZnS clusters and its aggregates act as effective photocatalysts in reduction of organic compounds. 3. The nanoclusters of cerium oxide (CeO2-x) materials have been found to possess a significant concentration of Ce3+ and oxygen vacancies resulting in excellent poisoning resistance against H2O and CO2 and substantial reduction in the temperature of selective SO2 reduction by CO. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Nanocrystalline Materials Applications of Nanocrystalline Materials 1. Crystalline nanoparticles used in a solar panel is claimed to have more efficiency and is more flexible and cheaper than many other panels. 2. Crystalline nanoparticles of zeolite are reportedly used as filter to convert crude oil to diesel fuel by a method cheaper than the conventional way. 3. Nanocrystalline and nanoporous Si exhibit electroluminescence, photoluminescence and refractive index changes that can be controlled. 4. The size control of nanoscale catalyst particles has been achieved in preparation of highly dispersed metal colloids and metal clusters fixed on to substrates by electrochemical reduction of metal salts. 5. Materials with higher hydrogen storage per unit volume and weight and considerably increased rate of hydrogen adsorption, like mixtures of nanoscale particles of Mg and Mg2Ni with high surface area, have been produced by mechanical means. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Fullerenes Two forms of fullerenes: (a) Bucky ball form; (b) nanotube form. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Buckyball clusters Nanotubes Megatubes Polyfullerenes Types of Fullerenes Endohedral fullerenes Exohedral fullerenes and heterofullerenes Nano-onions Fullerene rings Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Polyfullerenes Nano-onions Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Endohedral fullerenes Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Characteristics of Fullerenes 1. An important characteristic of C60 molecule is its high symmetry with 120 symmetrical operations which map the molecule onto itself. Each carbon atom bonds with three other adjacent atoms using sp2 hybridization. 2. Chemically fullerenes are stable; breaking the balls requires temperatures over 1000°C. However, fullerenes are not unreactive as they possess Π electrons which are free to localise or delocalise in different chemical situations, reactions such as addition reactions and redox reactions, are possible on their surface. This leads to covalent exohedral adducts and salts. 3. Fullerenes are insoluble in water, sparingly soluble in many other solvents and more soluble in toluene and carbon disulphide. 4. Fullerenes themselves are non-toxic but some of the derivatives of fullerenes could be harmful to the health. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Applications of Fullerenes 1. Oxidation involving free radicals causes cell damage, food spoilage, plastics deterioration, metal corrosion, etc. Therefore, fullerenes due to powerful antioxidant property (they reportedly react readily at a high rate with free radicals), can find application in health and personal care areas. 2. Fullerene nanotubes, which have small diameter and high aspect ratio, find use as ideal materials to be used as tips in scanning probe microscopy (SPM), such as scanning tunneling microscopy and atomic force microscopy. The nanotubes attached to the tip enhance SPM resolution to nanoscale as compared to the conventional silicon tip. 3. Fullerene nanotubes are promising as chemical molecular sensors, due to their small size, room temperature operation and high sensitivity. They find application as coating materials on some chemical sensors, such as quartz crystal microbalance and surface acoustic wave sensors (SAW). Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved 4. Field emission displays (FEDs) using carbon nanotubes are being explored by scientists which are expected to be better in terms of power, cost and view angles than the currently available flat panel displays, such as liquid crystal display (LCD) and plasma. 5. Fullerenes blended with polymers are used in organic photovoltaics. 6. Fullerenes can react readily with free radicals, thus preventing cell damage due to oxidation. They can hence be used as antioxidants. 7. Pharmaceutical companies are exploring the use of fullerenes in controlling Alzeimer’s and HIV. 8. Fullerenes and its modified forms are capable of targeted and controlled delivery of drugs and genes into cells. 9. Endofullerenes with their protected cage-structure are capable of being applied in MRI, X-ray imaging and radiopharmaceutical diagnostic applications. 10. Other applications of fullerenes like catalysts in certain organic reactions, water purification, fuel cell membrane material are also being explored. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Carbon Nanotubes Types of Carbon Nanotubes Single-walled nanotubes (SWNTs) Multiwalled nanotubes (MWNTs) Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Strength Hardness Characteristics of Carbon Nanotubes Kinetic Thermal Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Applications of Carbon Nanotubes 1. Carbon nanotubes find application in nanoelctronics. The nanotube integrated memory circuit was first made in 2004. CNT-based transistors can operate at room temperature and are capable of digital switching using a single electron. 2. A paper battery is made up of a paper-thin sheet of cellulose infused with aligned carbon nanotubes. The nanotubes act as electrodes to conduct electricity. These batteries provide a long and steady power output comparable to a conventional battery (lithium battery), as well as quick release of high energy (as a supercapacitor). 3. The solar cells have been developed at the New Jersey Institute of Technology using a carbon nanotube complex, formed by a mixture of carbon nanotubes and carbon buckyballs (fullerenes) to form snake-like structures. 4. Carbon nanotubes may be used to improve the efficiency of ultracapacitors. The activated charcoal used in conventional ultracapacitors has many small hollow spaces of various sizes, which create a large surface to store electric charge. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Other applications of carbon nanotubes include: 1. They have been introduced in nano-electromechanical systems, including mechanical memory elements and nanoscale electric motors. 2. CNTs find applications in composite polymer materials where they are used to further add to the strength of the polymers. 3. As CNTs have high mechanical strength, they are being explored for fabricating clothes’ stab-proof and bullet-proof properties. 4. A flywheel fabricated using CNTs, when spun at a very high velocity on a floating magnetic axis in vacuum, can store energy at a density approaching that of fossil fuels. 5. Due to their similarity in physical dimensions to those of biologically active macromolecules such as proteins and DNA, carbon nanotubes find increasing utility in biologically inspired design. Ultra-short SWNTs have been used as in vivo nanoscale capsules. In cancer research, SWNTs inserted around cancerous cells and excited with radio waves results in killing the cancerous cells. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Nanowires A nanowire is a wire with diameter of the order of a nanometer (10−9 meters) and an unconstrained longitudinal size. At these magnitudes since quantum mechanical effects are important, nanowires are also known as quantum wires. Applications of Nanowires 1. They find a lot of applications in the fields of electronics, optics, magnetic medium, sensor devices, etc. 2. They are used in the magnetic information storage medium 3. They find potential use in electronic applications such as junctions with good rectifying characteristics. Junction diodes, memory cells and switches, transistors, LEDs and inverter etc., have already been fabricated using nanowire junctions. 4. In biomedical field, nanowires are used to coat titanium implants which reduce the risk of implant failure. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Nanorods Nanorods are nanoscale objects with each of their dimension in the range 1 to 100 nm. These are produced directly by chemical synthesis using metals or semiconducting materials as starting material. Important examples are ZnO and gold nanorods. ZnO nanorods are generally prepared by vapor phase method. The vapors of ZnO may be obtained by any of the following three methods: 1. Chemical reduction: First ZnO is reduced to zinc metal vapors, transferred to growth zone and then reoxidized to ZnO vapors. 2. Thermal evaporation: Powdered ZnO (commercial grade) is mixed with SnO2 and heated to high temperature. 3. Vapor–liquid–solid method: Droplets of catalysts such as gold, copper, tin are deposited on a substrate and the gaseous mixture comprising Zn vapor and CO/CO2, reacts at the catalyst-substrate interface, followed by nucleation and growth. This method is most commonly used. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Applications of Nanorods 1. The main application of nanorods is in the area of display technologies because their reflectivity changes on change of orientation with an applied electrical field. They are also used in microelectromechanical (MEMS) systems. 2. These are conjugated with cancer therapeutic agents and ingested. Because they absorb near IR and generate heat when excited with IR radiation, nanorods selectively absorbed by the cancerous cells are heated on exposure to IR radiation and destroyed. 3. Nanorods fabricated using semiconducting materials are used in energy harvesting systems and light emitting diodes. ZnO nanorods are also extensively used to fabricate nanoscale electronic devices. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Dendrimers Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Applications of Dendrimers 1. Dendrimers are used in several biomedical applications. They are used in in vitro diagnosis of cardiac problems. They are used as contrast agents in preclinical studies for magnetic resonance (MRI). 2. In industrial processes, the combination of high surface area and high solubility makes dendrimers promising nanoscale catalysts. They have nanoscopic cavities which act like microenvironment for molecular reactions and reactor sites for catalysis. 3. Dendrimers with tailored solubility properties can find use in environment friendly industrial processes. 4. Amphiphilic dendrimer are useful in forming interfacial liquid membranes for stabilizing aqueous-organic emulsion. This helps in extracting chemical compounds between two phases. This arrangement holds promise for the development of organic chemistry in aqueous medium. 5. Dendrimers also find use in pH or proton sensors. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Nanocomposites A nanocomposite is a matrix reinforced by added nanoparticles. Nanocomposite is a multiphase solid material with one of the phases having dimensions <100 nm. The reinforcing material can be particles, sheets or fibres (e.g., carbon nanotubes). Due to the exceptionally high surface area to volume ratio of the reinforcing phase and/or its high aspect ratio, the area of the interface between the matrix and reinforcement phase(s) in nanocomposites is typically an order of magnitude greater than for conventional composite materials. Nanocomposites can be of following types: 1. Ceramic-matrix nanocomposites 2. Metal-matrix nanocomposites 3. Polymer-matrix nanocomposites Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Applications of Nanocomposites 1. In material science: • In production of batteries with greater power output. For example, silicon-carbon nanoparticles form anodes for lithium ion batteries. • In production of structural parts that have a high strength-to-weight ratio. • In making lightweight sensors with nanocomposites. • In making flexible batteries. • Due to improved mechanical properties, nanocomposites are used in automotive and industries involved in making door handles, engine covers, impellers and blades for vacuum cleaners, power tool housings and covers for portable electronic equipment such as mobile phones, etc. • Nanoclay incorporated materials act as barriers to gases. • Significant reductions in flammability and transmission of solvents through polymers incorporated with nanoclay fillers can result in the use of these materials as both fuel tank and fuel line components for cars. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved 2. 3. In life science • In replacement of tissues or tissue engineering (of skin, bone, cartilage, blood vessels) damaged by sickness or accidents. • In drug delivery. • In biosensor applications since core shell fibers of nanoparticles with fluid cores and solid shells can be used to entrap proteins, bacteria or viruses. The other future applications, owing to the possibility of building ordered arrays of nanoparticles in the polymer matrix, are in the manufacture of nanocomposite circuit boards, in neural networks applications, optoelectronics and optical computing and magneto-optical storage media manufacturing. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Biological Nanomaterials 1. Biological nanomaterials are biological systems which are inherently nano in size. They serve as models and help in understanding the behavior of nanomaterials in general and lead us to approaches like biomimicking. There are many nanophase materials in biological systems. 2. For example, living systems produce mineral material of the bone with particle size in the nanometer scale. These biological nanomaterials could even be used as direct source of novel materials or modified through in vivo procedures and in in vitro manipulations. 3. Some examples of biological nanomaterials are ferritins and related ironstorage proteins (for probing the magnetic properties of nanoscale materials and also to investigate iron metabolism in living organisms), nanoparticles found in magnetotactic bacteria (use earth’s magnetic lines to orient themselves and move in the direction of nutritional or chemical gradients), etc. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved General Applications of Nanomaterials The impact of nanotechnology and use of nano-materials extends from healthcare, medicine, energy, agriculture, food to fields such as electronics, communication, optics, chemistry and computing. In Medicine The size of nanomaterials is very similar to that of biological molecules and structures; therefore, these find extensive use in both in vivo and in vitro biomedical applications. The integration of nanomaterials with biology has led to development of various diagnostic devices, contrast agents, analytical tools, physical therapy applications and drug delivery vehicles. 1. Diagnostics: 2. Drug delivery 3. Tissue repair 4. Transdermal drug delivery Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved In Electronics and Communication Nanotechnology has brought revolution in telecommunication, computing and networking industries. The principle aim of nanotechnology is to produce three dimensional confined quantum structured electronic devices such as quantum wires and quantum dot devices. Some successful devices are: 1. Quantum well lasers for telecommunication. 2. High electron mobility transistors (HEMT) with low noise. 3. High gain microwave application and vertical cavity surface emitting lasers for data communication and sensor coding. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Some consumer products using nanomaterials are: 1. Computer hardware 2. Display devices. 3. Mobile and communication products. 4. Audio products. 5. Cameras and films, etc. Nanomaterials are used to develop semiconductors with much advanced properties. These include: 1. Transistors from carbon nanotubes with nanometer dimensions. 2. Memory chips with density of one tetrabyte per square inch. 3. Very high speed transistors from single atom thick graphene film. 4. Lightweight nanoemissive display panel using carbon nanotubes. 5. Nomfet (nanoparticle organic memory field-effect transistor) obtained by combining gold nanoparticles with organic molecules. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved In Energy Science Nanomaterials, particularly nanocomposites, are increasingly being used to replace conventional materials for conversion of energy. Some examples of their use are: 1. Non-porous crystalline solids called zeolites are used for refining of oil. 2. Quantum dots can be used as sources of light emissions. 3. Electrode materials can be changed by nano-structuring, that is, can be imparted nano-texture to improve electrical performance of batteries. 4. Energy production can be brought about using nanomaterials as clean and high efficiency energy sources. 5. Solar cells can be integrated from nanoscale crystals of semiconductors coated with light-absorbing dye-emitting electrons and from nanostructured diamond thermal cells that capture heat and light from the lattice and emit high-energy electrons. 6. Ultraporous nanomaterials are being explored for storing hydrogen at highdensities for fuel-cell powered cars. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved In Catalysis Heterogeneous catalysts used in industry today, consist of small particles (1-10 nm) of catalytically active material attached to porous support. Nanoparticles of metals, semiconductors, oxides, and other compounds are widely used for important chemical reactions. There are four main categories of nano-catalysts based on the composition. These include: 1. Gold-based (e.g., Au/TiO2, Au/MgO) 2. Other metal based (e.g., Cu, Rh,) 3. Platinum or palladium based (e.g., Pt/Ru/Ni) 4. Nanosize metal particles dispersed Pt/polypyrrole) in polymers (e.g., Cu/polyaniline, Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Miscellaneous Applications 1. 2. 3. 4. 5. 6. 7. Nano zinc oxide and titanium oxide are used in sunscreen creams, lotions and other cosmetics. These nanoparticles become transparent and retain their ability to absorb UV radiation. Nanoparticles of tungsten-carbide–cobalt provide a hard coating. This results in a greater resistance to wear in applications such as drill bits, cutting tools and jet engine parts. Nano coating over glass produces self-cleaning glass, which becomes transparent or opaque according to the current applied. Nanotechnology finds applications in energy storage and its conversion. Nanomaterials are investigated as electrode materials in batteries and fuel cells. Nanotubes also offer enhanced hydrogen storage capability for the use in fuel cells. Nanotechnology finds applications in the production, processing, safety and packaging of food stuffs. Nanofibers in clothes make waterproof and stain-repellant or wrinkle-free and can be washed less frequently. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved Future Perspectives of Nanochemistry Witnessing an exponential progress, nanotechnology is today among the fastest growing areas of science and technology. Nanotechnology can be expected to accomplish the following in the near future. 1. Create integrated circuits using three-dimensional carbon nanotubes contributing to the growth of computer power. 2. Design solar panels with greater efficiency using nanocrystalline materials. 3. Fabricate lighter and stronger military equipment using nanomaterial composites. 4. Revolutionise display technologies allowing bright images, light weight, less power consumption and wider viewing angles using nanostructured polymers. 5. Contribute to more efficient, energy saving chemical manufacturing processes with reduced waste by-products using nanostructured catalysts. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved 6. Protect humanity from ill health with reformulated pharmaceutical products using nanosized particle, improved targeted drug delivery, their administration and absorption, create tissue compatible implants and even build artificial organs. 7. Protect the environment from degradation by designing nanomembranes to capture carbon dioxide in the exhaust, using nanoparticles for effective cleaning up of organic solvents, using nanoclusters of silver for reducing polluting by products, using nanowires making alternate energy sources cost effective. 8. Make safe drinking water available to all at reasonable cost with water purification bottles, using filters only a few nanometres in width. 9. Produce smart materials with nanotechnology surfaces which are highly resistant to bacteria, dirt and scratches and fabrics that are highly resistant to liquid and stain-proof. 10. Nanotechnology is expected to continue the ever increasing miniaturization of semiconductor processing and memory devices. Engineering Chemistry (revised edition) ISBN: 978-81-265-4475-2 Copyright©2014 Wiley India Pvt. Ltd. All Rights Reserved