* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download RNA
Survey
Document related concepts
Transcript
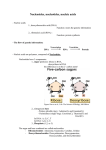
Nucleic acids and nucleoproteins The variety and complexity of functions that are carried out by proteins, in a number of cases demands, besides the amino acids residues presence, and other components. Depending on a chemical nature of non-protein component the conjugated proteins are subdivided on: chromoproteins (contains coloured component), glycoproteins (contains carbohydrates and their derivates), lipoproteins (contains lipids), phosphoproteins (contains a phosphoric acid), metalloproteins (contain various atoms of metals), and nucleoproteins (complexes of proteins and nucleic acids). Nucleic acid in nucleoprotein structure provides storage, realization and transfer of the hereditary information In 1868 the Swiss chemist F. Mischer for the first time extracted from human leucocite nuclei a new type of compound unknown before and named them nucleins (from lat. nucleus). Then nucleins were obtained from a nuclear material of various organisms. Later Mischer determined, that nuclein is a complex compound consisting of an acid component, phosphoruscontaining about 10 %, which was named nucleic acid, and protein component. So were discovered the nucleic acids and a new group of conjugated proteins nucleoproteins. In the middle 80es of ct. XIX nucleins were found in chromosome structure , in this connection the first representation about their important role in transfer of hereditary properties was generated. However these representations have not received further development, as transfer of genetic properties is connected with proteins. And only in the 50es of ct. XX. the convincing experimental proofs of a major role of the nucleic acids (DNA, RNA) in storage and transfer of a heredity were received. So it was proved, that nucleic acids are present in all cells of organisms, and are material carriers of the genetic information. Nucleic acids are linear directed heterobiopolymers, monomer parts of which are nucleotides . • Nucleotide - a compound that consists of nitrogencontaining base, carbohydrate component and a phosphoric acid residue. Nucleotides composition: 1. NITROGENOUS BASES Five bases are found in nucleic acids. Two purine bases (adenine and guanine) and three pyrimidine bases (cytosine, uracil and thymine). Adenine and guanine are present in both DNA and RNA. Cytosine and thymine are the pair of pyrimidines in DNA, and cytosine and uracil is the pair in RNA. 1 2 6 3 5 7 4 9 8 Purine 3 2 4 5 1 6 Pyrimidine Cytosine Uracil (in RNA) Thymine ( in DNA) 2. Carbohydrates (function - giving of hydrophylity): one of two pentoses - ribose (in RNA) or deoxyribose (in DNA). In nucleotides, both types of pentoses are in their β-furanose (closed five-member ring) form. 3. The residue of a phosphoric acid Н3РО4 (function the hinge connecting cyclic structures; Giving steady negative charge) Nucleoside=nitrogenous base+carbohydrate Purine bases between 9th nitrogen atom, and pyrimidine is linked through 1st – formed N-glycosidic bond with ribose or 2'-deoxyribose. Glycosidic bonds in nucleosides and nucleotides are always of the -configuration. Depend on pentose nature nucleosides are divided on ribonucleosides and deoxyribonucleosides. The names of nucleosides build of the trivial names according to nitrogenous base by addition suffix -idine at pyrimidinic, -osine - at purinic nucleosides: Name of nucleotide = name of base + -osine (purins) -idine (pirimidines) Glycosidic bond Cytidine Glycosidic bond Adenosine Nucleotides - phosphoric ethers of nucleosides. Usually in nucleosides esterified hydoxyle group at С-5 ' or at С-3 ' of pentose residue. Depending on a pentose structure are distinguished ribonucleotides and deoxyribonucleotides. Nucleotides is possible to consider, on the one hand, as ethers of nucleosides (their phosphates), and on the other hand - as acids, in connection with presence in their structure of the residue of a phosphoric acid. Name of nucleotide = name of nucleoside + number of phosphates Adenosine 5' monophosphate (АМP), adenylic acid Deoxythymidyine 5' monophosphate (ТМP), thymidylic acid Nucleoside 5'-Triphosphates are carriers of chemical energy Nucleoside 5'-triphosphates are indispensable agents in metabolism because the phosphoric anhydride bonds they possess are a prime source of chemical energy to do biological work. Adenosine 5'-triphosphate (ATP) has been termed the energy currency of the cell. Guanosine 5'triphosphate GTP is the major energy source for protein synthesis. Nucleic acids are linear polymers of nucleotides linked 3' to 5' by phosphodiester bridges. They are formed as 5'nucleoside monophosphates are successively added to the 3'-OH group of the preceding nucleotide DNA chain fragment Types of nucleic acids Deoxyribonucleic acid (DNA) - function is storage and transfer of the hereditary information Ribonucleic acid (RNA) - function is realization of the hereditary information Differences between DNA and RNA 1. In composition: 1.1. In nitrogenous bases: in RNA – uracile, in DNA – thymine. Cytosine Uracile Thymine 1.2. In carbohydrate component: in RNA – ribose, in DNA – deoxyribose. 2. In structure: most of RNA molecules – singlestrained, DNA- always double-stranded Two-chained DNA increases reliability of information storage , but results in necessity of RNA existence for its realization. 3. In cellular localization: The majority of DNA is concentrated in a nucleus, majority of RNA - in cytoplasm Deoxyribonucleic acid (DNA) Chargaff rules (1949 г.): [A] = [T]; [C] = [G]; [A] + [G]=[T]+[C] ([purines] = [pyrimidines]). [A] + [G]=[T]+[C] [aminogroups] = [oxogroups]. James Watson and Francis Crick, working in the Cavendish Laboratory at Cambridge Universityin 1953, took advantage of Chargaff’s results and the data obtained by Rosalind Franklin and Maurice Wilkins in X-ray diffraction studies on the structure of DNA to conclude that DNA was a complementary double helix. The specific pairing of the nitrogenous bases causes complementarity, I.e. supplementarity and interdependence of DNA strands each other. The nucleotids sequence in one polynucleotidic chain automatically determines a sequence of nucleotids in another, complementary strand. The two DNA strands are antiparallel : in one chain direction 51→ 31, in other – 31→ 51. Between planes of these pairs bases located the one above the other hydrophobic stacking interaction (from stacking laying in piles). DNA helix is usually rightinvolute The total material of chromosomes chromatine contains DNA, hystonic and non-hystonic proteins, small amount of RNA and ions of metals. Protein component of nucleoproteins 1. Structural proteins – positive charged, reached by diaminoacids 1.1. Histones: large (mass 15-20 thousands) Classes : Н1- reached by lysine, Н2А - reached by arginine and lysine, Н2В – modestly reached by arginine and lysine, Н3 – reached by arginine Н4 – reached by arginine and glycine. Functions: histones Н2А-Н4 form hystonic octamere, on which Is wound DNA, forming nucleosome; Histone Н1 connects separate nucleosomes together Protein components of nucleoproteins 1.2 Protamines – small (М 4-12 thousands) proteins, in which about 80% of amino acids is represented by arginine. Function - enter into nucleosome structure, filling space between histones 2. Regulatory proteins – negatively charged, reached by dicarboxylic amino acids – acid non-histonic proteins – protein factors of transcription and translation DNA occurs in various forms in different cells. The single chromosome of prokaryotic cells is typically a circular DNA molecule. Relatively little protein is associated with prokaryotic chromosomes. In contrast, the DNA molecules of eukaryotic cells, each of which defines a chromosome, are linear and richly adorned with proteins. Ribonucleic aсid (RNA) RIBOSOMAL RNA - the largest - structural and functional component of ribosomes Ribosomal RNAs characteristically contain a number of specially modified nucleotides, including pseudouridine residues, ribothymidylic acid, and methylated bases. Ribonucleic aсid (RNA) TRANSFER RNA Transfer RNA (tRNA) serves as a carrier of amino acid residues for protein synthesis. The tRNAs are the smallest RNAs (size range—23 to 30 kD) and contain 73 to 94 residues, a substantial number of which are methylated or otherwise unusually modified. . Unusual (minor) bases in tRNAs Ribonucleic aсid (RNA) . MESSENGER RNA Messenger RNA (mRNA) serves to carry the information that is encoded in genes to the sites of protein synthesis in the cell, where this information is translated into a polypeptide sequence. RNA copy is made of the sequence of bases along one strand of DNA. This mRNA then directs the synthesis of a polypeptide chain as the information that is contained within its nucleotide sequence is translated into an amino acid sequence by the protein-synthesizing machinery of the ribosomes.