* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download nucleic acid - Notes-for-all
Signal transduction wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Citric acid cycle wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
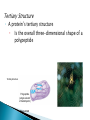
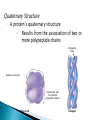
The Molecules of Cells Got Lactose? ◦ Many people in the world suffer from lactose intolerance Lacking an enzyme that digests lactose, a sugar found in milk ◦ Lactose intolerance illustrates the importance of biological molecules To the functioning of living cells and to human health 3.1 Life’s molecular diversity is based on the properties of carbon ◦ A carbon atom can form four covalent bonds Allowing it to build large and diverse organic compounds Structural formula Ball-and-stick model H H C Space-filling model H H H Methane C H H H The 4 single bonds of carbon point to the corners of a tetrahedron. Figure 3.1A Organic Compounds ◦ Carbon Based Molecules ◦ All Carbon Atoms have four places in which other atoms can bond A molecule composed of only Carbon and hydrogen. ◦ NON-POLAR MOLECULES Carbon Skeleton – hydrocarbons bonded together in a long chain. Isomer – a compound with the same formula but a different structure. ◦ Carbon chains vary in many ways ◦ http://bioweb.wku.edu/courses/BIOL115/Wyatt/ H H C C H H H H H H C C C H H H H H H Ethane Propane Carbon skeletons vary in length. H H H H H H H C C C C C H H H H H C C C H H H H H H H Butane Isobutane Skeletons may be unbranched or branched. H H H H H H H H H H C C C C H H C C C C H H H H H 1-Butene 2-Butene Skeletons may have double bonds, which can vary in location. H H C H H C H C H C C H C H H H H H H H H C C C C H C C H Benzene Cyclohexane Skeletons may be arranged in rings. Figure 3.1A H ◦ Functional groups are particular groupings of atoms That give organic molecules particular properties OH Estradiol HO Female lion OH O Figure 3.2 Male lion Testosterone A functional group is a group of atoms that usually participate in chemical reactions. 5 Functional groups include: Hydroxly Group - OH Carbonyly Group C=O Carboxyl Group COOH Amino Group NH2 Phosphate –OPO32- Hydroxly Group - OH ◦ Carbon chains with OH group form alcohols C=O group forms ◦ Ketones ◦ Aldyheydes COOH Groud form: ◦ Carboxylic Acids ◦ Acts as an acid by contributing H+ to solution and becoming ionized (takes on a charge). ◦ Example – Acetic Acid (aka Vinegar) NH2 Groups are known as amines. ◦ Form Amino acids ( the building blocks of proteins) a.a. are a combination of the amine group NH2 and Carboxyl Group COOH. OPO32- Usually bound to a carbon skeleton by the Oxygen – this functional groups is involved with energy production (ATP) 3.2 Functional groups help determine the properties of organic compounds ◦ Examples of functional groups Table 3.2 ◦ The four main classes of Macromolecules are: Carbohydrates Lipids Proteins nucleic acids Macromolecules ◦ Large molecules Polymers ◦ Cells make most of their molecules by joining organic molecules into chains. Monomers ◦ The unit that serves as the building blocks of poymers Proteins are nothing more than amino acids strung together. Carbohydrates are sugars strung together. Lipids (fats) are glycerol chains bound to fatty acids Nucleic Acids are a series of four different bases (A, G, C, & T) arranged into a double helix. ◦ Cells make most of their large molecules By joining smaller organic molecules into chains called polymers ◦ Cells link monomers to form polymers By a dehydration reaction H OH OH OH Short polymer Unlinked monomer Dehydration Dehydratio reaction n reaction H2O OH O H H H Longer polymer Figure 3.3A H H ◦ Polymers are broken down to monomers by the reverse process: HYDROLYSIS H2O H OH Hydrolysis H OH OH Figure 3.3B H How many molecules of water are needed to completely break down a polymer that is 50 monomers long? How many molecules of water are needed to completely break down a polymer that is 50 monomers long? 49 Re-read 3.1 – 3.3 and fill in notes. Complete Chapter Objective 3.1 – 3.3 Prepare for quiz 3.1 – 3.3 ◦ On-Line Book Support Activities 3A – 3C ◦ Key Concept Quiz Read 3.4 – 3.7 Carbohydrates The term carbohydrates refers to a class of molecules that range from: small sugar molecules (sugar in soft drinks) To large polysaccharides (starch molecules in potatoes/pasta) Are monosaccharides Figure 3.4A Monomers: ◦ Single unit sugar Honey consists of the monomers glucose and fructose ◦ A monosaccharide has a formula that is a multiple of CH2O and contains hydroxyl groups and a carbonyl group. Example: Glucose has the formula C6H12O6 ◦ The monosaccharides glucose and fructose are isomers That contain the same atoms but in different arrangements C H C OH C O C H H C OH HO C H H C OH H C OH H C OH H C OH H C OH H C OH H Figure 3.4B H O H Glucose HO H Fructose ◦ Monosaccharides can also occur as ring structures 6 CH2OH H 5C CH2OH O H H H C 1 4C OH OH 3C H OH O H OH H OH HO C2 H H H O OH OH Structural formula Figure 3.4C Abbreviated structure Simplified structure 3.5 Cells link two single sugars to form disaccharides ◦ Monosaccharides can join to form disaccharides Such as sucrose (table sugar) and maltose (brewing sugar) CH2OH CH2OH O O H HO H H H OH H H OH HO OH H H H OH Glucose OH H OH Glucose H2O CH2OH H HO Figure 3.5 CH2OH O H OH H H OH H H O Maltose O H OH H H OH H OH 3.6 How sweet is sweet? ◦ Various types of molecules, including nonsugars Taste sweet because they bind to “sweet” receptors on the tongue. Table 3.6 3.7 Polysaccharides are long chains of sugar units ◦ Polysaccharides are polymers of monosaccharides Linked together by dehydration reactions. ◦ Starch is a stored polysaccharide in plants. It consists entirely of glucose monomers that form a helix. ◦ Starch (potatoes) and glycogen (sugar stored in animals) are polysaccharides that store sugar for later use. ◦ Cellulose is a polysaccharide found in plant cell walls and is the most abundance organic compound on earth. O O O O O GLYCOGEN O O Cellulose fibrils in a plant cell wall O O O O O O O O O O CELLULOSE OO OO O OH OO O OH OO O OO Figure 3.7 O O O O Cellulose molecules O O O Glycogen granules in muscle tissue Glucose monomer STARCH Starch granules in potato tuber cells OO OO O O O O O O Unlike glycogen and starch, most animals cannot break down by hydrolysis (insoluble fiber). ◦ Cows and termites have cellulose-hydrolyzing microorganisms that can digest cellulose. Almost all carbohydrates are ◦ Hydrophilic (water loving), thus cotton bath towels are quite absorbant. Re- read 3.4 – 3.7 Carbohydrates Fill in notes Prepare for quiz 3.4 – 3.7 ◦ Complete Chapter Objective 3.3 – 3.7 ◦ On-line book support 3D & 3E ◦ Key Concept Quiz Read 3.8 – 3.10 ◦ Lipids are diverse compounds That consist mainly of carbon and hydrogen atoms linked by nonpolar covalent bonds. ◦ Lipids are grouped together Figure 3.8A Because they are hydrophobic (water fearing) ◦ Fats, also called triglycerides Are lipids whose main function is energy storage. Consist of glycerol linked to three fatty acids H H H C C OH OH Figure 3.8B H H C H OH Glycerol HO C O H2O CH2 CH2 CH2 CH2 CH2 Fatty acid CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 Figure 3.8C H H H C C C O O O C O C O C H O CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 CH3 CH CH2 CH2 CH2 CH2 CH2 CH2 CH3 ◦ Phospholipids are a major component of cell membranes. ◦ Waxes form waterproof coatings ◦ Steroids are often hormones H3C CH3 CH3 HO Figure 3.9 CH3 CH3 3.10 Anabolic steroids pose health risks ◦ Anabolic steroids Are synthetic variants of testosterone Can cause serious health problems Compromises your immune system Can alter cholesterol levels Depression and mood swings Increase blood pressure Infertility Interfers with bone growth Re- read 3.8 – 3.10 Lipids Fill in notes Prepare for quiz 3.8 – 3.10 ◦ Complete Chapter Objective 3.8 – 3.10 ◦ On-line book support 3F ◦ Key Concept Quiz Read 3.11 – 3.16 ◦ A protein Is a polymer constructed from amino acid monomers held together by peptide bonds. They are thus referred to as: polypeptides. There are 20 different amino acids Proteins form different shapes, based upon the order that the amino acids are strung together. ◦ Proteins Are involved in almost all of a cell’s activities ◦ As enzymes They regulate chemical reactions. Figure 3.11 3.12 Proteins are made from amino acids linked by peptide bonds ◦ Protein diversity Is based on different arrangements of a common set of 20 amino acid monomers. ◦ Each amino acid contains An amino group A carboxyl group An R group, which distinguishes each of the 20 different amino acids H O H N C H C OH R Amino group Figure 3.12A Carboxyl (acid) group ◦ Each amino acid has specific properties Based on its structure H H H O N H C CH2 O N CH CH3 H C OH H C H O C N OH H OH CH2 CH2 OH C Hydrophobic Figure 3.12B OH Serine (Ser) C H CH3 Leucine (Leu) C O Aspartic acid (Asp) Hydrophilic ◦ Cells link amino acids together By dehydration synthesis ◦ The bonds between amino acid monomers Are called peptide bonds Carboxyl group Peptide bond Amino group H H H O N H C C H + OH O N C Dehydration reaction H C H N OH R R Amino acid Amino acid H2O H H O C C R H N C H R Dipeptide Figure 3.12C O C OH 3.13 A protein’s specific shape determines its function ◦ A protein consists of one or more polypeptide chains Folded into a unique shape that determines the protein’s function Groove Figure 3.13A Groove Figure 3.13B 3.14 A protein’s shape depends on four levels of structure Primary Structure ◦ A protein’s primary structure Is the sequence of amino acids forming its polypeptide chains Levels of Protein Structure Leu Met Pro Primary structure Gly Thr Gly Glu Cys Ser Lys Asn Val Val Lys Val Ala Leu Asp Ala Val Arg Gly Ser Pro Amino acids Figure 3.14A Ala Ile Val His Val Phe Arg Secondary structure ◦ A protein’s secondary structure Is the coiling or folding of the chain, stabilized by hydrogen bonding Amino acids Hydrogen bond C C N H O C Secondary structure C O C N H N H O C C C C H O N H O C C N N H O C N H O C R C H C N H O O C C C N H Alpha helix Figure 3.14B O H H O N C CN H R CC N C CN H CC O H O O H O C N CC N C C H H O C C N CN H O C C C N H O H O N C CN H CC N C C N H C O H O O H O C N CC N H C N C H O C CN H O C Pleated sheet Tertiary Structure ◦ A protein’s tertiary structure Is the overall three-dimensional shape of a polypeptide Tertiary structure Polypeptide (single subunit of transthyretin) Figure 3.14C Quaternary Structure ◦ A protein’s quaternary structure Results from the association of two or more polypeptide chains Polypeptide chain Quaternary structure Transthyretin, with four identical polypeptide subunits Figure 3.14D Collagen 3.19 Linus Pauling contributed to our understanding of the chemistry of life ◦ Linus Pauling made important contributions To our understanding of protein structure and function Figure 3.15 3.20 Nucleic acids are information-rich polymers of nucleotides ◦ Nucleic acids such as DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell ◦ The monomers of nucleic acids are nucleotides Composed of a sugar, phosphate, and nitrogenous base H H N N N H OH O P N O CH2 Nitrogenous base (A) O O Phosphate group H H H H OH Figure 3.16A Sugar N H H ◦ The sugar and phosphate form the backbone for the nucleic acid or polynucleotide A T C G T Figure 3.16B Sugar-phosphate backbone Nucleotide ◦ DNA consists of two polynucleotides Twisted around each other in a double helix C A C C T G G A T C G A T T A Base pair G T A A T Figure 3.16C A C T ◦ RNA, by contrast Is a single-stranded polynucleotide ◦ Stretches of a DNA molecules are called genes: Genes are the amino acid sequences that code for proteins.