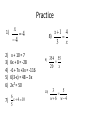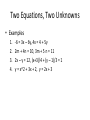* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Math review (1 OF 2) 2 equations w 2 unknowns
Large numbers wikipedia , lookup
Elementary algebra wikipedia , lookup
Principia Mathematica wikipedia , lookup
Bra–ket notation wikipedia , lookup
Abuse of notation wikipedia , lookup
Elementary mathematics wikipedia , lookup
History of algebra wikipedia , lookup
Positional notation wikipedia , lookup
Musical notation wikipedia , lookup
Honors Physics Math Review Key Math Skills Needed for Success! • Conversions – Know metric conversion factors – Know how to properly convert from one unit to another • Scientific Notation – How to convert between scientific notation and standard notation • Algebra – Solve for x • Geometry/Trig – SOHCAHTOA Conversions • Metric Base units (100) – Definition: Fundamental unit – Mass: gram (g) – Length: meter (m) – Time: seconds (s) – Temperature: kelvin (K) – Amount of substance: mole (mol) – Volume: Liter (L) Conversions • Prefixes – Greater than base unit • • • • Deca- (101), Hecto- (102), Kilo- (103) Deca- = ten, Hecto- = hundred, Kilo- = thousand Abbreviations: Deca da, Hecto h, Kilo k Example: 1 hectometer is the same as saying 100 meters or 102 meters – Less than base unit • • • • Deci- (10-1), Centi- (10-2), Milli- (10-3) Deci- = 1/10, Centi- = 1/100, Milli- = 1/1000 Abbreviations: Deci d, Centi c, Milli m Example: 1 centimeter is the same as saying one onehundreth of a meter or 10-2 meters Conversions • Conversion factors – All conversion factors must equal 1 – Conversion factors take the form of a fraction – Just as 5/5, 28/28, and 142/142 all equal 1, there are multiple ways of writing conversion factors that equal 1. – Example: __100 cm__, __1 km__, __1000m__ 1m 1000m 1 km Conversions • Conversion Factors – How to read them • Example: __1000 mm__, there are 1000 1m millimeters in 1 meter. • Ask yourself, “Does this make sense?” – How to properly write them • The smaller unit always gets the number greater than 1 Practice • Write the conversion factors you would use to convert from… – cm to m – m to cm – kg to g – g to kg – ms to s – s to ms Conversions • How do I know which unit goes in the numerator and which unit goes in the denominator when making my conversion factor? – The unit that you are converting FROM always goes in the denominator – The unit you are converting TO always goes in the numerator Practice • Convert the following (make sure to properly write your conversion factor): – 42 cg = ? g – 6 m = ? km – 132 ms = ? s – 86 kg = ? g Conversions • What if I am not converting to my base unit? For example, 58 cm = ? km – First convert your know quantity to your base unit • 58 centimeters equals how many meters • 58 cm = .58 m – Second convert your base unit to your desired unit • .58 meters equals how many kilometers • .58 m = .00058 km Practice • • • • • 9 mm = ? cm 18 kg = ? dag 95 mg = ? kg 63 cg = ? hg 1,468 dm = ? km Scientific Notation • Scientific notation makes the expression of very large or very small numbers simpler. • Makes it easier to keep track of significant figures. • In Physics, you will deal with very large numbers such as the distance from the sun to the Earth which is 149,600,000,000 meters. Scientific Notation • General form: a x 10n – a must be a number between 1 and 10 – n must be an integer – Example: These are NOT in scientific notation • 34 x 105… Why? • 4.8 x 100.5… Why? Scientific Notation • What’s the difference between a positive exponent and a negative exponent? – Positive exponents tell you how many times to multiply by 10 – Negative exponents tell you how many times to divide by 10 Scientific Notation • Converting from standard form to scientific notation – Remember… a x 10n – Move the decimal point left or right until you wind up with a number between 1 and 10 • The number you are left with is “a” – The number of spaces the decimal point is moved is the exponent “n” Scientific Notation • Converting from standard notation to scientific notation…how do I know if my exponent is positive or negative? – If the decimal is moved to the left, you will have a positive exponent • In other words, “a” is less than the number you started with – If the decimal is moved to the right, you will have a negative exponent • In other words, “a” is greater than what you started with Practice • Write 3,040 in scientific notation • Write 0.00012 in scientific notation • Write 149,600,000,000 in scientific notation – The distance from Earth to the sun • How many kilometers are in 1 meter? (write the answer in scientific notation) • How many milligrams are in 1 kilogram? (write the answer in scientific notation) Scientific Notation • Converting from scientific notation to standard form – Remember… a x 10n – If “n” is positive, move the decimal point in “a” to the right • In other words, if “n” is positive your answer will be greater than your original “a” – If “n” is negative, move the decimal point in “a” to the left • In other words, if “n” is negative your answer will be less than your original “a” Scientific Notation • How to enter a number in scientific notation into your calculator. – Remember… a x 10n – Enter “a” – Press the “EE” button – Enter “n” – Do NOT press the multiplication button or enter the number 10 • “EE” takes the place of this step Practice • Write the following in standard form – 4.01 x 102 – 5.7 x 10-3 – 8.9 x 105 – 6 x 10-1 Algebra • Solving for “x” • Key things to remember – What you do to the left side of the equation you MUST do to the right side of the equation. – What you do to one term you must do to ALL terms on both sides of the equation • Think back to… order of operations – Please excuse my dear aunt sally – Parentheses exponents multiply divide add subtract – Left to right Algebra • Solving for “x” • First combine all like terms abiding by order of operations – Remember… • variables do NOT mix with non-variables • 1x + 1x = 2x, 3x + 4x = 7x • x(x) = x², 2x(6x) = 12x², 2x(3x²) = 6x³ – Example: 3x + 14 – 5x + x = 2 + 1 + 3(5) – 6x 14 – x = 18 – 6x Algebra • Solving for “x” • Second, move all terms containing a variable to one side of the equation and all other terms to the other side. • Remember… – Opposite of addition is subtraction and vice versa – Opposite of multiplication is division and vice versa • Example: 14 – x = 18 – 6x 5x = 4 Algebra • Solving for “x” • Finally, make it so that the coefficient of “x” is 1 – This means divide both sides by the coefficient of “x” – If there are multiple terms on the opposite side, make sure to divide each term by the coefficient – Example: 5x = 4 x = 4/5 Algebra • If the variable is in the denominator use cross multiplication • For example… 1. 54 72 d 64 2. y 15 35 y4 7 Algebra • Solving for “x” – If your “x” term is squared then make your last step to take the square root of both sides of the equation – Remember… • you can plug your answer back into the equation – If the left side of the equation equals the right side then you solved for “x” correctly! Practice 1) 2) 3) 4) 5) 6) x 4 4 x + 10 = 7 6x + 8 = -28 -6 + 7x +3x = -116 6(3-x) = 48 – 3x 2x² = 50 6 x 4 10 7) 5 x 1 4 8) 3 x 9) 10) 354 55 20 x 3 5 w6 w4 Two Equations, Two Unknowns • System of Equations: set of 2 or more equations that use the same variables. 2x – y = 7, 4x + 3y = 4 • Solve by substitution: 1. Solve for one of the variables. 2x – y = 7 y = 2x - 7 Two Equations, Two Unknowns 2. Substitute the expression for the variable that you solved step 1 for into the second equation. 4x + 3y = 4 4x + 3(2x – 7) = 4 x = 2.5 3. Substitute the value of x into either equation. y = 2x – 7 y = 2(2.5) – 7 y = -2 Quadratic Equation • When solving an equation in the form, ax^2 + bx + c = 0, you may need to use the quadratic formula if reverse FOIL does not work Two Equations, Two Unknowns • Examples 1. 2. 3. 4. -6 = 3x – 6y, 4x = 4 + 5y 2m + 4n = 10, 3m + 5 n = 11 2x – y = 12, (x+3)/4 + (y – 1)/3 = 1 y = x^2 + 3x + 2, y = 2x + 3