* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 4.Electron e/m ratio
Survey
Document related concepts
Transcript
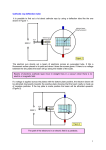
1: 2: (ta initials) first name (print) last name (print) brock id (ab13cd) (lab date) Experiment 4 Electron charge-to-mass ratio In this Experiment you will learn • the relationship between electric and magnetic fields as applied to an electron; • how to determine the strength of the Earth’s magnetic field; • to compensate for the effect of undesirable contributions in an experiment; • to extend your data analysis capabilities with a computer-based fitting program; • to apply different methods of error analysis to experimental results. Prelab preparation Print a copy of this Experiment to bring to your scheduled lab session. The data, observations and notes entered on these pages will be needed when you write your lab report and as reference material during your final exam. Compile these printouts to create a lab book for the course. To perform this Experiment and the Webwork Prelab Test successfully you need to be familiar with the content of this document and that of the following FLAP modules (www.physics.brocku.ca/PPLATO). Begin by trying the fast-track quiz to gauge your understanding of the topic and if necessary review the module in depth, then try the exit test. Check off the box when a module is completed. FLAP PHYS 1-1: Introducing measurement FLAP PHYS 1-2: Errors and uncertainty FLAP PHYS 1-3: Graphs and measurements FLAP MATH 1-1: Arithmetic and algebra FLAP MATH 1-2: Numbers, units and physical quantities WEBWORK: the Prelab Electron Test must be completed before the lab session Important! Bring a printout of your Webwork test results and your lab schedule for review by the ! TAs before the lab session begins. You will not be allowed to perform this Experiment unless the required Webwork module has been completed and you are scheduled to perform the lab on that day. Important! Be sure to have every page of this printout signed by a TA before you leave at the end ! of the lab session. All your work needs to be kept for review by the instructor, if so requested. CONGRATULATIONS! YOU ARE NOW READY TO PROCEED WITH THE EXPERIMENT! 30 31 The electron charge-to-mass ratio This experiment, performed in 1897 by J.J. Thomson, demonstrated that atoms are not fundamental units of matter but are composed of aggregates of charged particles, protons and electrons. It was used to determine a value for e/m, the ratio of electron charge to electron mass. Commonly called the Bainbridge Experiment in honour of the scientist who developed the method, this experiment exhibits an ingenious combination of electromagnetic theory and mechanics. Figure 4.1: The Bainbridge Tube, with front at bottom and base at left As shown in Figure 4.1, the central component of the apparatus is a large vacuum tube (Bainbridge tube). Inside the tube, a horizontal metal cylinder with a narrow opening at the conical end, called the anode, encloses an axially mounted wire, the cathode. The cathode heater power supply provides a voltage across the cathode wire and causes a significant electron current flow through the wire. Collisions between these energetic electrons and the atoms in the wire ejects some electrons from the material, forming an electron cloud inside the cyclinder, around the cathode wire. The anode power supply is then used to set the anode at a potential V more positive than that of the cathode, to create a radial electric field inside the cylinder that will accelerate the negatively charged electrons toward the outer anode. Some of these electrons shoot through the slot in the anode. After escaping, they will continue to move in a straight line with a constant speed v since there is no electric field outside the anode. Work-energy theorem demands that the work done by the electric field in accelerating an electron is equal to the change in the electron’s potential energy, ∆(K.E.) = W = eV. (4.1) Assuming that the initial kinetic energy of the electrons near the surface of the cathode is zero, Equation 4.1 yields the value for the kinetic energy an electron has after it escapes through the slot in the anode. 32 EXPERIMENT 4. ELECTRON CHARGE-TO-MASS RATIO Changing the anode voltage V controls this kinetic energy and, therefore, the speed v of the electron, 1 K.E. = mv 2 2 ⇒ v= r 2V e . m (4.2) The second stage of this experiment involves magnetic forces. Two large Helmholtz coils, positioned above and below the Bainbridge tube, are used to create a uniform magnetic field in the tube. With this ~ is vertical, perpendicular to the velocity ~v arrangement, the direction of the magnetic field intensity (B) of the electrons. The magnetic force (F~ ) experienced by an electron is given by the vector cross-product ~ F~ = e~v × B and F = F~ = evB. (4.3) ~ is always perpendicular to ~v , it does no work on the electron, and cannot change its kinetic energy Since F or its speed. The magnetic force acts as a centripetal force, Fc = mac , making the electrons move in a ~ From mechanics, the centripetal force is circle of radius r. The plane of the circle is perpendicular to B. F = evB = Fc = mac = mv 2 . r (4.4) Rearranging Equation 4.4 so that only e/m remains on the left-hand side yields e 2V = 2 2. m r B (4.5) Thus the charge-to-mass ratio for an electron, e/m, can be determined from V , r and B. The magnetic field strength B depends of the radius R and the number of turns N of wire of the Helmholtz coils and the current I flowing in them: 8µ0 N B= √ I 5 5R (4.6) where B is in units of Tesla (T), I in units of Ampere (A) and µ0 = 4πx10−7 (T-m/A2 ) is the magnetic constant of a vacuum. For the Helmholtz coils of this apparatus, N = 150 turns and R = 0.140 m, then B = (9.634 × 10−4 ) I The Banibridge tube is filled with mercury vapour. Electrons colliding with mercury atoms induce these atoms to emit a faint blue-green light, making the electron path visible in a dark room. By varying the magnetic field strength B at constant anode voltage V , or by varying V at constant B, the electron beam is made to follow a circular path. A horizontal scale with a sliding pointer is used to measure the left and right extremes of this circular beam. This yields tha beam diameter from which the beam radius can be determined. This is essentially the strategy we will pursue for this experiment: set a current I and voltage V , determine the size of the circular orbit r, determine the magnetic field strength B from Equation 4.6, then substitute these values into Equation (4.5) to calculate the ratio e/m. The electron beam emerging from the electron gun may spread out slightly as it goes around the orbit. There are two main reasons for this spreading. First, not all the electrons leave the slot with exactly the ~ Also, collisions speed v given by Equation (4.2), nor are all of the velocities ~v exactly perpendicular to B. with the mercury may change the velocity of the electrons and therefore, the radius of their orbit. 33 Figure 4.2: Schematic Diagram of Electrical Apparatus Procedure The experimental electronics are shown in Figure 4.2. Ask the instructor to explain the operation of any of the electrical components which you do not understand. 1. The Accelerating Voltage dial sets the accelerating potential V applied to the electrons inside the cylinder and hence determines the velocity v of the electrons emitted from the tip of the electron gun. Once outside the gun, the speed of the electrons will not change. The Accelerating Voltage, measured in Volts, is displayed on the analog meter labelled V. To see a beam, V must be ≈ 80 V or greater, otherwise the electrons will not have enough energy to ionize the Mercury gas in the Bainbridge Tube and make it glow. The analog meters used to measure current and voltage also operate on a magnetic principle: here a coil is mounted on concentric pivots between the poles of a permanent magnet, with a pointer mounted in the plane of rotation. When a current flows through the coil, a magnetic field is produced that interacts with the magnetic field of the permanent magnet, causing the coil and attached pointer to rotate. A circular scale can then display the current flowing through the coil. To take a reading from an analog meter, view the needle at a right angle to the face of the meter so that it’s profile will be minimized, then the point on the scale behind the needle represents the best value for the measurement. 2. The Magnetizing Current dial adjusts the amount of current I flowing through the Helmholtz coils and hence their magnetic field intensity B. This current, measured in Amperes, is displayed on the analog meter labelled A. 3. The power supply that sends a current through the cathode filament in order to heat it and make it and release electrons, sets the beam intensity and is not adjustable in this experiment. The measurement error of an analog meter is equal to one-half of the smallest increment in the in the meter scale. Note: There are no workstations in the darkroom. As you proceed to do Parts 1-3 of this Experiment, begin by performing all the the data gathering steps in the darkroom with the Bainbridge Tube, then migrate to a workstation to complete the calculations, graphing and analysis part of the Experiment. 34 EXPERIMENT 4. ELECTRON CHARGE-TO-MASS RATIO Part 1: Initial setup and precautions With the power supply set to ”OFF”, perform the following steps: • set the Deflecting Voltage polarity to ”OFF” and voltage control fully counterclockwise to minimum. This voltage, when applied to a pair of plates inside the electron gun, will cause the beam to bend. This control is not part of the experiment; • set the Accelerating Voltage control fully counterclockwise to minimum; • set the Magnetizing Current direction to ”OFF” and current control fully counterclockwise to minimum. • Turn the power supply to ”ON”. Verify that the filament inside the electron gun illuminates. Allow the cathode to heat up for a few minutes before applying Accelerating Voltage. During the operation of the tube, observe the following precautions: • bring the Accelerating Voltage up slowly; • do not allow the electron beam to strike the glass envelope of the tube for an extended period of time. This happens when there is an applied Acelerating Voltage and insufficient Magnetizing Current to bend the beam in a circular path inside the tube; • limit the operating session to one hour to prevent the overheating of the electron gun; • turn all controls to minimum and all switches to ”OFF” before turning the power ON or OFF! Part 2: Constant anode voltage In this section you will measure the diameter of the electron beam as you vary the magnetic field while keeping the accelerating voltage constant. You will then determine the average he/mi and the standard deviation σ(e/m) of the distribution of e/m values calculated from this experimental data. Similarly, you will determine hvi and σ(v) from the set of v results. • With the tube warmed, slowly increase the Accelerating Voltage to 150 V. A straight beam should emerge from the electron gun. • Set the current direction to clockwise, then increase the Magnetizing Current I to 1.0 A; the electron beam should now trace a circular orbit. • Position your eye directly in front of the left side of the beam’s arc. Slide the vertical pointer along the horizontal scale until it is directly in line with the left side of the arc. When your eye, pointer and beam edge are correctly aligned, the pointer will appear to have a minimum width and will overlap the edge of the beam. • Record the index position d1 in Table 4.1, then repeat the above step for the right side of the beam and record this index position d2 in the data table. • With the radius of the beam r given by r = (d2 − d1 )/2, calculate r, then using Equation calculate e/m. Record Im and V in the appropriate row of Table 4.1. Calculate below the values of B, r, e/m and the speed v of the electrons, then enter the results in Table 4.1. r= d2 − d1 2 = ............................ = .................. 35 B = (9.634 × 10−4 ) I = ............................ = .................. 2V = ............................ = .................. v= rB 2V e = 2 2 = ............................ = .................. m r B • Repeat the above measurements and calculations for Magnetizing Currents of 1.2, 1.4, 1.6, 1.8, 2.0 and 2.2 A, keeping V constant. i V (V) I (A) 1 150 1.0 2 150 1.2 3 150 1.4 4 150 1.6 5 150 1.8 6 150 2.0 7 150 2.2 d1 (m) d2 (m) r (m) B (T) v (m/s) e/m (C/kg) Table 4.1: Calculation of e/m and electron velocity v Since there is a sample of several values for v and e/m, the best error estimate σ is obtained from a statistical calculation that yields the averages hvi, he/mi and standard deviations σ(v), σ(e/m). The standard deviation of a sample σ provides a measure of how closely the sample values are clustered about the sample average. i 1 e m e e m− m e 2 e m− m i 2 3 3 4 4 5 5 6 6 7 7 variance = (v − hvi)2 v − hvi 1 2 e m = e= σ m v hvi = variance = σ(v) = Table 4.2: Calculation of hxi and σ(x) for e/m and v. Here, variance(x) = σ 2 (x) = 1 N −1 PN i=1 (xi − hxi)2 . 36 EXPERIMENT 4. ELECTRON CHARGE-TO-MASS RATIO • Use Table 4.2 as your worksheet to calculate the average and the standard deviation of e/m and v. • In Physicalab, click File, New to clear the data window, then enter in a column the seven e/m values. Select Options, Insert X index to insert a column of index values. • Check bellcurve y data to display a distribution of your e/m values and the Gaussian bellcurve determined from the average and standard deviation of the sample. The value of he/mi ± σ(e/m) should be identical with the results that you obtained in Table 4.2. Email yourself a copy of the graph. • Repeat the above step using the seven values for v. You may now complete this part of the experiment by reporting the final results, correctly rounded, for e/m and v. e/m = .................. ± .................. C/kg v = .................. ± .................. m/s Part 3: Constant coil current In this section you will determine e/m and σ(e/m) by performing a linear least squares fit on a set of graphed data points. Equation (4.5) can be rewritten to indicate that V varies with r 2 . 2 B2 e 2 B e V = r = r2 (4.7) 2 m 2m and is the equation of a straight line through the origin, with B 2 e/(2m) as the slope. To test this relation, I and hence B will be fixed and V varied to to change the beam diameter. • Set the Magnetizing Current to 2.0 A and V to 100 V to produce a visible beam. ? As the voltage is decreased below 100 V, how does the beam behave? Why is this so? At what voltage is the beam no longer visible? • Determine the beam radius for V = 100 − 220 V and enter the results in Table 4.3. • Enter in the Physicalab data window the coordinate pairs (r 2 , V ). Select scatter plot. Click Draw to generate a graph of your data. Your data should approximate a straight line. If this is not the case, check your data and then redraw the graph. • Select fit to: y= and enter A*x+B in the fitting equation box. Here B is the fitting parameter corresponding to the Y-intercept of the straight line, not the magnetic field vector B. Label the axes and title the graph. Click Draw , then Send to: to email the group a copy of the graph. • From the fit, record below the value of the slope A and error ∆(A): A= B 2e = .................. ± .................. V/m2 2m (4.8) • Determine ∆(I) by using the instrument scale error, then B, ∆(B), e/m and ∆(e/m). Finally, summarize these results in the proper format: 37 i V (V) I (A) 1 100 2.0 2 120 2.0 3 140 2.0 4 160 2.0 5 180 2.0 6 200 2.0 7 220 2.0 d1 (m) d2 (m) r (m) r 2 (m) Table 4.3: Data for determining e/m and σ(e/m) using a linear least squares fit ∆(I) = ........................ = ...................... = ...................... B = ........................ = ...................... = ...................... ∆(B) = ........................ = ...................... = ...................... e/m = 2 ∗ A ∗ B −2 = ...................... = ...................... s e ∆A 2 ∆(B) 2 + = ...................... = ...................... ∆(e/m) = m A B I = .................. ± .................. A B = .................. ± .................. T e/m = .................. ± .................. C/Kg Part 4: electron e/m From the Internet or another source, or using the known values of e and m, determine the value of e/m. e/m = .................. IMPORTANT: BEFORE LEAVING THE LAB, HAVE A T.A. INITIAL YOUR WORKBOOK! 38 EXPERIMENT 4. ELECTRON CHARGE-TO-MASS RATIO Lab report Go to your course homepage on Sakai (Resources, Lab templates) to access the online lab report worksheet for this experiment. The worksheet has to be completed as instructed and sent to Turnitin before the lab report submission deadline, at 11:00pm six days following your scheduled lab session. Turnitin will not accept submissions after the due date. Unsubmitted lab reports are assigned a grade of zero. ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ 39 ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................ ................................................................................