* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Management of Chemotherapy-Induced Cardiotoxicity in Female
Remote ischemic conditioning wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Coronary artery disease wikipedia , lookup
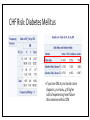
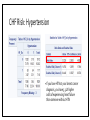
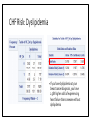
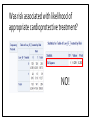
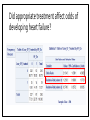
MANAGEMENT OF THERAPY-INDUCED CARDIOTOXICITY IN FEMALE BREAST CANCER PATIENTS Hailey Baker, Tamara McMahon, Carol Fabian, Bruce Kimler, Russ Waitman Background • Increasingly more women are surviving from breast cancer due to effectiveness of biologics, chemotherapies, and new radiation technology • Focus can no longer be solely on survival, but now much concern chronic quality-oflife issues • Cardiovascular health is of particular concern in the United States • American Society of Clinical Oncology (ASCO) guidelines (August 2016) • Unknown if clinicians follow and how that affects patient outcomes Specific Aims • Aim 1: Characterize KUMC breast cancer population • Aim 2: Did patients receive appropriate screening for their risk category (based on ASCO guidelines)? • Aim 3: Explore potential risk factors for heart failure after cardioabrasive chemo or radiotherapies • Aim 4: Investigate whether appropriate cardioprotective agents prevent heart failure Inclusion & Exclusion Criteria: • Females • Breast cancer diagnosis • • • SEER Site Summary ICD9 Code 174 ICD10 Code C50 • Diagnosed after 01/01/2008 • Class of Case 14 • Initial diagnosis at KUMC and all of first course treatment or a decision not to treat was done at KUMC. TOTAL = 1632 patients HERON Cohort: • Extent of disease (e.g. stage, ER/PR/HER2 status) • Underlying risk factors for cardiovascular (CV) disease • • Smoking, hypertension, diabetes, dyslipidemia, and obesity Cancer Treatment • • Cardiotoxic chemotherapy (e.g. anthracyclines, Trastuzumab, kinase inhibitors) Radiation therapy where the heart is in the treatment field • Cardiovascular screening (e.g. Echocardiogram) • CV Outcomes: Heart failure, low LVEF • Prevention Therapy • BB, ACEI, or ARBs High Risk • High dose anthracycline (e.g. ≥250 mg/m2 doxorubicin, ≥600 mg/m2 epirubicin) • High dose (≥30 Gy) radiotherapy where the heart is in the treatment field • Lower dose anthracycline (e.g. <250 mg/m2 doxorubicin, <600 mg/m2 epirubicin) in combination with lower dose radiotherapy (<30 Gy) where the heart is in the treatment field • Treatment with lower dose anthracycline (e.g. <250 mg/m2 doxorubicin, <600 mg/m2 epirubicin) or trastuzumab alone, and presence of any of the following risk factors: • • • • Multiple (≥2) cardiovascular risk factors, including: smoking, hypertension, diabetes, dyslipidemia, obesity during or after completion of therapy Older (≥60 years) age at cancer treatment Compromised cardiac function (e.g. borderline low LVEF [50-55%], history of myocardial infarction, ≥moderate valvular heart disease) at any time prior to or during treatment Treatment with lower dose anthracycline (e.g. <250 mg/m2 doxorubicin, <600 mg/m2 epirubicin) followed by trastuzumab (sequential therapy) Else = LOW RISK AIM 1: CHARACTERIZE POPULATION Patient Demographics: Language English P = 0.1899 Patient Demographics: Race P = 0.8874 White Patient Demographics: Marital Status W D Single Married P = 0.3448 Patient Characteristics: Vital Status Alive Dead Dead Alive P = 0.0003 Patient Characteristics: Stage P < 0.0001 Patient Characteristics: BC Type P < 0.0058 Patient Characteristics: Chemotherapy P < 0.0001 Total = 384 patients Patient Characteristics: Radiotherapy < 3,000 cGY = Low Dose > 3,000 cGY = High Dose Total = 566 P < 0.0001 Patient Characteristics: Co-Morbidities Risk Age at Dx Smoke Diabetes HTN Dyslipid. Prior MI Total 0 61 (± 12) 88 (9.2%) 78 (8.1%) 210 (21.9%) 153 (16.0%) 2 (0.21%) 959 1 59 (± 11) 48 (7.2%) 44 (6.6%) 154 (23.0%) 140 (20.9%) 1 (0.15%) 670 Total ------ 136 122 364 293 3 1,629 P-value 0.0034 0.15 0.24 0.60 0.024 0.46 ------ Patient Characteristics: BMI P-value = 0.2876 Aim 1: Conclusions • Demographics: English-speaking white married women who are primarily still living with mostly stage 1-2 ER+PR+HER-breast cancer • Higher mortality in low-risk population • Unequal proportions of breast cancer stages between risk groups • • Treatment Characteristics: The majority of patients who received chemotherapy were treated with anthracyclines or Trastuzumab • The majority of patients who received radiation were high-risk patients given high-dose radiotherapy to the breast • More high-risk women received anthracyclines than low-risk women • • Co-Morbidities: Low-Risk = slightly older & higher rates of dyslipidemia • Otherwise, very similar demographics and characteristics • AIM 2: DID PATIENTS RECEIVE APPROPRIATE SCREENING FOR RISK CATEGORY? ASCO Guidelines: Pre-Treatment • Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline • “Clinicians should perform a comprehensive assessment in cancer patients that includes a history and physical examination, screening for cardiovascular disease risk factors (hypertension, diabetes, dyslipidemia, obesity, smoking), and an echocardiogram prior to initiation of potentially cardiotoxic therapies.” • Recommendations as of 15 August 2016 Pre-Treatment Screening Frequency Table: Interpretation: • Chi-Square p-value < 0.001 • If you’re at higher risk of CV disease, you have 5.66X higher odds of receiving pretreatment screening with an echo Pre-Treatment LVEF by Risk Category ASCO Guidelines: During Treatment • Recommendation 4.3 • Routine surveillance imaging may be offered during treatment in asymptomatic patients considered to be at increased risk (Recommendation 1.1) of developing cardiac dysfunction. In these individuals, echocardiography is the surveillance imaging modality of choice that should be offered. Frequency of surveillance should be determined by healthcare providers based upon clinical judgment and patient circumstances. • • Recommendation 4.4 • No recommendations can be made regarding continuation/discontinuation of cancer therapy in individuals with evidence of cardiac dysfunction. This decision, made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances, and considering the risks/benefits of continuation of therapy responsible for the cardiac dysfunction. • • (Evidence-based; Benefits outweigh harms; Evidence quality: Intermediate; Strength of Recommendation: Moderate) (Informal consensus; Benefits outweigh harms; Evidence quality: Insufficient) Recommendation 4.5 • Clinicians may use routine echocardiographic surveillance in patients with metastatic breast cancer continuing to receiving trastuzumab indefinitely. The frequency of cardiac imaging for each patient should be determined by healthcare providers, based upon clinical judgment and patient circumstances. • (Evidence-based and Informal Consensus; Benefits outweigh harms; Evidence quality: Low; Strength of Recommendation: Moderate) Screening Intervals P < 0.0001 0.0009 0.0197 0.0484 0.1673 Change in LVEF by Screening Intervals Aim 2: Conclusions • Pre-Treatment Echo: • • • Most patients are receiving appropriate pre-treatment screening echocardiograms for their respective risk category. Women at higher risk were more likely to receive pre-treatment echocardiograms. However, there is still room for improvement because there were 23% of high risk women that did not receive pre-treatment echocardiograms During Treatment Echo: • • • High-risk patients receive significantly more echocardiograms before and after treatment through 9 months Biggest drop in LVEF occurs between months 3 and 6 for low-risk women Biggest drop in LVEF occurs between months 0 and 3 and months 9 and 12 for high-risk women AIM 3: EXPLORE POTENTIAL RISK FACTORS FOR HEART FAILURE IN BC PATIENTS CHF Risk: Diabetes Mellitus • If you have DM at your breast cancer diagnosis, you have 4.47X higher odds of experiencing heart failure than someone without DM CHF Risk: Hypertension • If you have HTN at your breast cancer diagnosis, you have 3.32X higher odds of experiencing heart failure than someone without HTN CHF Risk: Dyslipidemia • If you have dyslipidemia at your breast cancer diagnosis, you have 2.58X higher odds of experiencing heart failure than someone without dyslipidemia CHF Risk: Smoke Status • If you smoke at your breast cancer diagnosis, you have 3.03X higher odds of experiencing heart failure than someone who doesn’t smoke CHF Risk: Family History of Heart Disease • If you have a family history of CV disease at your breast cancer diagnosis, you have 1.59X higher odds of experiencing heart failure than someone who doesn’t have a family history of heart disease CHF Risk: Risk Category • If you have a higher risk of CV disease at your breast cancer diagnosis, you have 0.62X lower odds of experiencing heart failure than someone who is lower risk Aim 3: Conclusions Risk Factor Rank of Risk Factor Risk Odds Ratio Diabetes Mellitus 1 4.47 Hypertension 2 3.32 Smoking Status 3 3.03 Dyslipidemia 4 2.58 Family History 5 1.59 Risk Category 6 0.62 • Diabetes Mellitus is the most significant risk factor for experiencing CHF • Women at higher risk may be monitored more closely, which prevents experiencing heart failure in the future AIM 4: INVESTIGATE WHETHER APPROPRIATE CARDIOPROTECTIVE AGENTS PREVENT CHF Were patients treated appropriately? NOTE: only 67 patients in the entire cohort received a heart failure ICD9 or ICD10 code Was risk associated with likelihood of appropriate cardioprotective treatment? NO! Did appropriate treatment affect odds of developing heart failure? Aim 4: Conclusions • The majority (86%) of patients who had a LVEF < 50% were not prescribed cardioprotective medications (BB, ACEI, or ARB) within 30 days of their abnormal echo result • Risk at diagnosis did not affect a woman’s odds of receiving appropriate treatment at CHF diagnosis • Unable to conclude with certainty whether treatment with cardioprotective medications affects your odds of developing heart failure • May be underdiagnosing heart failure in breast cancer survivors, which could skew results Overall Conclusions • Aim 1: Characterize breast cancer population • • Similar characteristics between high and low-risk cohorts Aim 2: Did patients receive appropriate screening for their risk category? Most patients are receiving appropriate pre-treatment screening echocardiograms for their respective risk category. • High-risk patients receive significantly more echocardiograms before and after treatment through 9 months • • Aim 3: Explore potential risk factors for heart failure after cardioabrasive chemo or radiotherapies Diabetes mellitus was the most significant risk factor for higher odds of developing CHF • High risk appeared to be protective for CHF, possibly due to closer clinical follow-up • • Aim 4: Investigate whether appropriate cardioprotective agents prevent heart failure • Most patients did not receive cardioprotective chemotherapies when their LVEF < 50% Future Directions • No recommendation can be made on the risk of cardiac dysfunction in cancer patients with any of the following treatment exposures: • • • • Lower dose anthracycline (e.g. <250 mg/m2 doxorubicin, <600 mg/m2 epirubicin) or trastuzumab alone, and no additional risk factors (as defined in 1.1) Lower dose radiotherapy (<30 Gy) where the heart is in the treatment field, and no additional cardiotoxic therapeutic exposures or risk factors (as defined in 1.1) Kinase inhibitors No recommendations can be made regarding the use of cardioprotective strategies (dexrazoxane, continuous infusion, liposomal formulation) in patients receiving lower (e.g. <250 mg/m2 doxorubicin, <600 mg/m2 epirubicin) cumulative dose of anthracyclines. QUESTIONS?? Thanks!