* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Current Status of Pneumonia and Influenza Diagnostics
Surround optical-fiber immunoassay wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Trichinosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Hepatitis C wikipedia , lookup
Oesophagostomum wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Swine influenza wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Neonatal infection wikipedia , lookup
Schistosomiasis wikipedia , lookup
Gastroenteritis wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Leptospirosis wikipedia , lookup
Hepatitis B wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Neisseria meningitidis wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
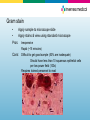
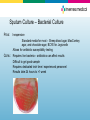
Diagnostic Testing for Community-Acquired Pneumonia (CAP) and Influenza Norman Moore, Ph.D. Director of Clinical Affairs [email protected] Objectives • Discuss the etiological agents for pneumonia and which age groups are most prone to the infection. • Describe what clinical samples should be taken and how they should be transported to the laboratory for analysis • State the diagnostic testing methods recommended for community-acquired pneumonia and influenza • Show how influenza can lead to pneumonia Infectious Disease in the US 1970: William Stewart, the Surgeon General of the United States declared the U.S. was “ready to close the book on infectious disease as a major health threat”; modern antibiotics, vaccination, and sanitation methods had done the job. 1995: Infectious disease had again become the third leading cause of death, and its incidence is still growing! Pneumonia is the sixth leading cause of death in the US and the major cause of death from infectious disease in the US. Current Number of Pneumonia Cases (US) • 37 million ambulatory care visits per year for acute respiratory infections (physician and ER visits combined) • Community-Acquired Pneumonia (CAP) – Each year 2 - 3 million cases of CAP result in ~ 10 million physician visits & 500,000 hospitalizations in the US – Average mortality is 10-25% in hospitalized patients with CAP • Nosocomial Pneumonia – Standard definition: onset of symptoms occurs approx 3 days after admission – 250,000 - 350,000 cases of nosocomial pneumonia per year – 25 - 50% mortality rate Etiological Agents • Newborns (0 to 30 days) – Group B Streptococcus, Lysteria monocytogenes, or Gram negative rods are common – RSV in premature babies • Infants and toddlers – 90% of lower respiratory tract infections are viral with the most common being RSV, Influenza A&B, and parainfluenza. Bacterial infections are rare, but could be S. pneumoniae, Hib, or S. aureus. Etiological Agents • Outpatient – S. pneumoniae, H. influenzae, M. pneumoniae, C. pneumoniae, and respiratory viruses • Inpatient (non-ICU) – With the above agents, add L. pneumophila • Inpatient (ICU) – S. pneumoniae, S. aureus, L. pneumophila, Gramnegative bacteria, and H. influenzae Streptococcus pneumoniae • Types – Over 90 serotypes exist, with 88% of disease covered in the 23-valent vaccine • Incidence – 100,000 to 135,000 cases of pneumonia requiring hospitalization up to the year 2000 – Around 80% of CAP – Cases are dropping due to the S. pneumoniae vaccine • Transmission – Person to person • Risk groups – The young and elderly • Most common identification – Blood culture and sputum culture Haemophilus influenzae • Types – The original risk was H. influenzae Type B (Hib), but vaccine has dramatically reduced pneumonia due to Hib, but other types and non-typeable strains still cause disease • Incidence – Variable • Transmission – Person to person • Risk groups – The young and elderly • Most common identification – Blood culture and sputum culture Chlamydia pneumoniae • Incidence – Overall is unknown, but in the literature, it seems to go in cycles so high incidence in some years and low in others. – Can be considered 3rd most common etiological agent in respiratory tract infections of young adults behind Mycoplasma pneumoniae and influenza • Transmission – Person to person • Risk groups – All age groups, but more common in school-age children. • Most common identification – Serology Personal contact with Barry Fields – Chief of Respiratory infections from CDC – rates of C. pneumoniae have been extremely low for years and he currently doesn’t view this as a significant infection. Mycoplasma pneumoniae • Incidence – Estimated 2 million cases and 100,000 pneumonia related hospitalizations in US • Transmission – Person to person by respiratory secretions, usually close contact – Outbreaks in crowded conditions like military and college which can last several months • Risk groups – All age groups, but more common in school-age children and young adults. – Most common etiological agent for adults younger than 30 • Most common identification - Serology Legionella pneumophila • Incidence – Estimates vary greatly from 15,000 per year to 100,000 per year in US • Transmission – Contaminated water – Outbreaks in hospitals, ships, hotels, etc. • Risk groups – Usually elderly, smokers • Most common identification – Urinary antigen Viral pneumonia • Adults may get viral pneumonia by “influenza, adenovirus, cytomegalovirus, parainfluenza, varicella, rubeola, or respiratory syncytial virus, particularly during epidemics” – Viral pneumonia, especially influenza, may cause a secondary bacterial disease, such as pneumococcal pneumonia Influenza A&B • Impact of influenza in the US – Hospitalizations up from 114,000 to 226,000 – 36,000 deaths annually – Influenza target population: 188MM in US • 5-20% of US population affected by influenza each year • Most deaths affect elderly and young children – Also affects otherwise healthy individuals Influenza Treatment • Antiviral drugs are available – Must be administered within 48 hr of onset of symptoms – Generally reduce duration of symptoms by one day – First generation drugs (amantidine, rimantidine) are cheaper but only treat influenza A – Second generation drugs (Tamiflu®, Relenza®) are more expensive but treat both influenza A and B – Reason to differentiate between influenza A and B Respiratory Syncytial Virus • Almost all children with have RVS by their second birthday – 25% to 40% will have signs or symptoms of bronchiolitis or pneumonia – 0.5% to 2% will require hospitalization – Recovery is in 1 to 2 weeks, but they can spread virus for 1 to 3 weeks • The elderly can get a usually mild RSV infection due to a weakened immune system – Rapid tests are not recommended on this population Specimen Collection Swab collection • Swab should remain moist and cultured within 4 hours • If longer than 4 hours to get to culturing, should use transport medium • Refrigeration, not frozen Sputum Collection • Quality of specimen – Care should be taken in collection since a lower respiratory tract sample can be contaminated with upper unless collected by an invasive technique • Collection – Patient is instructed to give a deep coughed specimen • Put into sterile container, trying to minimize saliva • Transport to lab immediately – Patient unable to give specimen can be given an aerosolinduced specimen Blood culture • Usually done with fever spike • Standard is to take two sets of blood cultures one hour apart Urine • Urine can be used for Legionella and Streptococcus pneumoniae – Antigen test – Noninvasive sample – Does not need to be qualified like a sputum sample Influenza Sample Collection • Appropriate specimens – Nasal wash/aspirate, nasopharyngeal swab, or nasal swab – Throat swabs have dramatically reduced sensitivity • Samples should be collected within first 24 to 48 hours of symptoms since that is when viral titers are highest and antiviral therapy is effective • Testing can be done immediately with rapids or sample placed in transport media – Infectivity is maintained up to 5 days when stored @ 48°C – If the sample cannot be evaluated in this time period, the sample should be frozen @ -70°C. Diagnostic Methods Available Infectious Disease Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults (2007) • Diagnostic Testing – Suggestive clinical features combined with a chest radiograph or other imaging technique is required for the diagnosis of pneumonia – It is recommended that “patients with CAP should be investigated for specific pathogens that would significantly alter standard (empirical) management decisions, when the presence of such pathogens is suspected on the basis of clinical and epidemiologic clues.” Infectious Disease Society of America/American Thoracic Society CAP Guidelines 2007 • When to apply diagnostic tests – Optional for outpatients with CAP – Blood culture and sputum culture for inpatients with productive cough* – All adult patients with severe CAP, should have blood culture, sputum culture, Legionella urinary antigen and S. pneumoniae urinary antigen tests* Common Diagnostic Tests • • • • • • • • Gram stain Sputum culture Blood culture Latex agglutination assays DFA/IFA PCR Serology Urinary antigen Gram stain • Apply sample to microscope slide • Apply stains & view using standard microscope Pros: Inexpensive Rapid (~15 minutes) Cons: Difficult to get good sample (50% are inadequate) Should have less than 10 squamous epithelial cells per low power field (100x) Requires trained personnel to read Sputum Culture – Bacterial Culture Pros: Inexpensive Standard media for most – Sheep blood agar, MacConkey agar, and chocolate agar, BCYE for Legionella Allows for antibiotic susceptibility testing Cons: Requires live bacteria – antibiotics can affect results Difficult to get good sample Requires dedicated tech time / experienced personnel Results take 24 hours to >1 week Legionella Culture • Legionella – Legionella needs specific growth conditions • Buffered charcoal yeast extract (BCYE) plate • Clinical sample may need to be acid treated to reduce general microflora • May take 3 to 10 days to get result Cell culture for Chlamydia pneumoniae • Chlamydia cultures should be transported in 2-sucrose phosphate or other transport medium • Use HeLa cell line rather than McCoy that is used for C. trachomitis • May take 3 to 10 days and is labor-intensive Culture for Mycoplasma pneumoniae • Specialty media • May take over 3 weeks for result – Vial is inspected daily and is prone to contamination (usually indicated by color shift in first 5 days) – Needs subculturing to agar – Highly labor intensive Blood Culture Pros: Inexpensive Allows for antibiotic susceptibility testing High specificity Cons: Requires live bacteria – antibiotics can affect results Requires dedicated tech time / experienced personnel Results take 24 hours to >1 week Many bacterial infections don’t progress to bacteremia Latex Agglutination • • Detecting antigen associated w/certain serogroups Polystyrene latex particles coated with antibodies Pros: Cons: Relatively simple Rapid (~15 minutes) Does not detect all serogroups of S. pneumoniae Procedure associated with urine is cumbersome Interpretation of results can be subjective Fluorescent Antibody (DFA/IFA) • Performed directly from sample on microscope slide – • Sputum, pleural fluid, aspirated material, or tissue Add fluorescent-tagged antibody specific for specific bacteria Observe for fluorescence using a special microscope Pros: Relatively quick turn around time (~1 hour) Cons: More labor intensive than rapid tests Requires trained technologist and special microscope Few labs equipped to perform DFA on 2nd/3rd shifts Sensitivity can be poor (25% to 75% on Legionella) Polymerase Chain Reaction (PCR) • • Molecular technique using a clinical sample Extract and amplify nucleic acid (DNA or RNA) of specific pathogen Pros: Extremely sensitive – can detect one microorganism Detects both live and dead pathogens Cons: Requires highly trained technologist, expensive equipment More labor intensive than rapid tests Prone to cross-contamination (false positives) Serology • Chlamydia pneumoniae – Measurement usually of acute and convalescent serum • A four-fold rise in titer is considered diagnostic – A single IgM titer of 16 or greater or IgG of 512 or greater is considered suggestive of recent infection • Mycoplasma pneumoniae – A fourfold rise from acute to convalescent serum or complement fixation titer of 1:128 in single serum specimen Urinary antigen • Tests are available for S. pneumoniae and L. pneumophila serogroup 1 • With Legionella, antigen appears in the urine 1 to 3 days after infection • Noninvasive sample • Easy-to-use Test Procedure for Urinary Antigen • • • • • • • • Collect urine sample (no pre-treatment i.e. concentrating, boiling, filtering, etc.) Open device pouch and lay flat Dip provided sampling swab into urine Place swab in lower hole of swab well and push up Add required number of drops of Reagent A (2 drops for Legionella test and 3 for S. pneumoniae) Close device Wait 15 minutes Interpret results Diagnostic Methods for Influenza • • • • Culture DFA PCR Rapid Tests Viral Culture • Pro – Highly sensitive as long as sample is properly handled • Con – Can’t give same day result to help monitor therapy – High level of difficult/equipment DFA • Pro – Usually considered to have high level of sensitivity – Can usually test for other respiratory pathogens at the same time – Results can be achieved in same day • Con – Labor intensive needed experienced users – Turn-around time from lab usually takes many hours PCR • Pro – For respiratory specimens, high performance – Same day results • Con – Turn around time from lab is extensive, especially if batching specimens – Expensive – Requires experienced technicians, labs, dedicated equipment, etc. Rapid Tests • Pro – Tests take minimal time – Some tests are so simple that they can be CLIAwaived – Can be used to triage patients – Positive results can be used to rule out other issues like pneumonia so don’t give unnecessary chest x-ray, antibiotics, etc. • Con – Performance is not as good as culture, PCR, and DFA The Connection Between Influenza and S. pneumoniae Pandemic outbreaks • In 1957 and 1968 influenza pandemic outbreaks, it was shown that a bacterial agent was present in approximately 70% of the serious (life-threatening or death) cases. • In contrast, in non-pandemic years, only 25% of serious cases had a secondary bacterial infection. Synergy Between Influenza and S. pneumoniae • Influenza neuraminidase found to prime lung for S. pneumoniae invasion. – S. pneumoniae has its own neuraminidase that it uses to promote binding to cells. – In a mouse model, if neuraminidase inhibitors were added, then mortality went down. • Recombinant versions of influenza strains of past 50 years were made. – 1957 and 1997 pandemic strains that were related to bacterial pneumonia had highest levels of neuraminidase activity. S. pneumoniae and Penicillin Penicillin Breakpoint Minimum Inhibitory Concentration (MIC) (mcg/mL) Susceptible (S) Intermediate (I) Resistant (R) Updated ≤2 4 ≥8 Previous ≤0.06 0.12-1.0 ≥2 • IV Penicillin – Less expensive than broad spectrum antibiotics – Reduce broad spectrum antibiotic resistance – Less liver/kidney resistance Reference • Mandell, L.A., R.G. Wunderink, A. Anzueto, J.G. Bartlett, G.D. Campbell, N.C. Dean, S.F. Dowell, T.M. File, D.M. Musher, M.S. Niederman, A. Torres, and C.G. Whitney. “Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults.” Clinical Infectious Diseases. 2007; 44:S27-72. • Murray, P.R., E.J. Baron, J.H. Jorgensen, M.A. Pfaller, and R.H. Yolken. Manual of Clinical Microbiology 8th Edition. • Forbes, B.A., D.F. Sahm, and A.S. Weissfeld. Bailey & Scott’s Diagnostic Microbiology 12th Edition.