* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHAPTER 11 BONDING AND MOLECULAR STRUCTURE:
Elias James Corey wikipedia , lookup
Kinetic resolution wikipedia , lookup
Homoaromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Aromaticity wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Aromatization wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
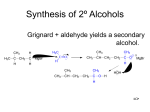
CHAPTER 11 BONDING AND MOLECULAR STRUCTURE: ORGANIC CHEMISTRY All bold numbered problems. Overview • Organic chemistry is the study of compounds containing carbon combined with other non-metals. • Bonding plays a critical role in understanding the reactivity of these compounds. • These compounds are referred to as hydrocarbons since they are primarily hydrogen and carbon. Overview Overview • Carbon uses sp3, sp2, and sp hybridization in forming the four bonds per carbon atom common to almost all carbon compounds. • With sp hybridization, there are two (2) p bonds and 1 s bonds. • With sp2 hybridization there is one (1) p bond and 1 s bonds. Why Carbon sp3, sp2, and sp hybridization Allotropes of Carbon Carbon only (no other atom) compounds importance FUNCTIONAL GROUPS 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Alkane Alkene Organic chemistry is the study of Alkyne compounds containing carbon. The goal of studying Organic Alkyl halide chemistry is the making of Aromatic carbon-carbon bonds, C-X, C-O, C-N, and C-S bonds to make new molecules Alcohol Ethers Aldehyde Ketone Carboxylic Acid Inorganic molecules like CO, and Ester -2 are not considered organic CO 3 Amine molecules. Amide 1. Alkanes Figure 11.4 10 Structural Formula BP (C) MP (C) 1 CH4 -161 -183 ethane 2 CH3CH3 -88 -172 propane 3 CH3CH2CH3 -45 -187 butane 4 CH3CH2CH2CH3 -.5 -138 pentane 5 CH3CH2CH2CH2CH3 36 -130 hexane 6 CH3CH2CH2CH2CH2CH3 69 -95 heptane 7 CH3CH2CH2CH2CH2CH2CH3 98 -90 octane 8 CH3CH2CH2CH2CH2CH2CH2CH3 125 -57 nonane 9 CH3CH2CH2CH2CH2CH2CH2CH2CH3 151 -54 decane 10 CH3CH2CH2CH2CH2CH2CH2CH2CH2CH 175 -30 Name # of C's methane 3 Alkanes 12 Abbreviated, common method to write organic cmpds Name methane Structural Formula C CH4 ethane CH3CH3 propane CH3CH2CH3 butane CH3CH2CH2CH3 pentane CH3CH2CH2CH2CH3 ALKANES • There are frequently many hydrocarbons with the same formula. These are called structural isomers. C5H12 has three isomers C10H22 has 75 isomers C20H42 has 366,319 isomers Isomers of Butane-5 Carbon chains C6H14 has five isomers, draw them. Cycloalkanes • Cycloalkanes are ring structures and have the general formula CnH2n. • Do not confuse these compounds with the alkenes which have the same general formula. 2. ALKENE AND 3. ALKYNE • These compounds are referred to as unsaturated. • Alkenes contain one or more double bonds and alkynes contain one or more triple bonds. Saturated –vs- Unsaturated Saturated Hydrocarbon C15H32 Unsaturated Hydrocarbon Contains either double and/or triple bonds Notice how the chains do not line up 21 Single bonds only Fats Double bonds Fats ALKENES ALKYNE Physical properties change Isomers have different physical as well as different chemical properties Nomenclature • Organic Functional Groups and Nomenclature • Substituents - saturated carbon substituents are called alkyl groups and are named based on the naming of the normal alkanes. H methy l C H CH3 Me H e t hyl H H C C H H H CH2C H3 Et Nomenclature Haloalkane 27 Alcohol 28 Ether CH3 CH2 O CH2 CH3 29 Amine 30 Aldehyde O CH3 C H 31 Ketone 32 Carboxylic acid O CH3 C OH 33 Ester O CH3 C O CH3 34 Amide 35 Draw the following • • • • • • Butane Butene Butyne Butanol Butanal Butanone • • • • • • Dibutyl ether Butanoic acid Ethyl butanoate Butyl amine Dibutyl amine Tributyl amine Common Alcohol Names What is the name of this Alcohol? alcohol methyl Methanol The main chain is numbered such that the first substituent encountered along the chain receives the lowest possible number. CH 3 C 1 C 2 C 3 C 4 C 5 2-methylpentane CH 3 C 5 C 4 NOT C 3 4 -m ethylpent ane C 2 C 1 If two or more identical substituents are attached to the same C-chain , prefixes di-, tri-, tetra-, etc. are used with numbers to indicate position. CH 3 C 1 C 2 CH3 C 3 C 4 C 5 2 ,4- dimethylpent ane CH3 C 1 2 C C 3 C 4 C 5 CH3 2, 2-dimethylpentane 40 Quiz Chapter 4 Section 3 Provide the IUPAC names of the alkanes below. CH3 CH3CH2CHCH2CHCH3 CH2CH3 3,5-dimethylheptane (CH3)2CHCH2CH(CH3)2 2,4-dimethylpentane If two different substituent's are attached to the carbon chain, name them in alphabetical order. CH CH 2 C 1 C 2 C 3 CH 3 C 4 C 5 3 C 6 C 7 3-ethyl, 5-methylheptane Numbering starts at the side with the heavier functional Group Numbering starts closest to 1st functional group Practice C C C C C C C C C 3 C C 2 C 4 C 5 C 6 C 1 3-ethylhexane C –Nomenclature of Branched Alkyl Chains » Two alkyl groups can be derived from propane 43 –Nomenclature of Branched Alkyl Chains » Four groups can be derived from the butane isomers 44 44 45 Examples 46 • Classification of Hydrogen Atoms » Hydrogens take their classification from the carbon they are attached to 46 47 Quiz Chapter 4 Section 3D Name the alkane below and identify as 1o, 2o and 3o , all groups of equivalent H. 1o 2o CH3CH2CHCH2CH3 CH3 1o 3-methylpentane 3o 48 –Nomenclature of Alkyl Halides » In common nomenclature the simple haloalkanes are named as alkyl halides • Common nomenclature of simple alkyl halides is accepted by IUPAC and still used 48 Practice Br C C Cl C C C 2-bromo-4-chloropentane Br C C Br C C Br 2,2,4-tribromopentane C 50 – IUPAC Substitutive Nomenclature » An IUPAC name may have up to 4 features: locants, prefixes, parent compound and suffixes » Numbering generally starts from the end of the chain which is closest to the group named in the suffix – IUPAC Nomenclature of Alcohols » Select the longest chain containing the hydroxyl and change the suffix name of the corresponding parent alkane from -ane to -ol » Number the parent to give the hydroxyl the lowest possible number » The other substituents take their locations accordingly 50 Common Names of simple alcohols are still often used and are approved by IUPAC 51 52 Common Names of Alcohols Alkyl group names are approved by IUPAC for naming alcohols: "alkyl group + alcohol." CH3CH2OH Ethanol CH3 CH3CCH2OH CH3 OH CH3CHCH3 2 propanol or isopropanol Neopentylol or 2,2-Dimethyl-1-propanol C 53 » Alcohols with two hydroxyls are called diols in IUPAC nomenclature and glycols in common nomenclature 53 Name the following compound. CH3CHClCH2CHOH CH3 4-chloro-2-pentanol Draw 54 55 Nomenclature of Cycloalkanes Cyclic alkanes are named with the "cyclo" prefix followed by the alkane name indicating the number of carbon atoms in the ring. CH2 CH2 CH2 cyclopropane CH2 CH2 CH2 CH2 cyclobutan e 56 • Nomenclature of Cycloalkanes –The prefix cyclo- is added to the name of the alkane with the same number of carbons • When one substituent is present it is assumed to be at position one and is not numbered • When two alkyl substituents are present the one with alphabetical priority is given position 1 • Numbering continues to give the other substituent the lowest number • Hydroxyl has higher priority than alkyl and is given position 1 • If a long chain is attached to a ring with fewer carbons, the cycloalkane is considered the substituent 57 Substituted Cycloalkanes The name of a substitutent is added as a prefix to the cycloalkane name. Alkyl group names are used for simple alkyl group substituents. When there are two or more substituents, the positions around the ring are numbered beginning with the substituent first in the alphabet. CH2CH3 Cl CH3 CH3 CH3 ethylcyclohexane 1,3-dimethylcyclohexane 1-chloro-2-methylcyclopentane 3 The cycloalkane also can be named as a substituent on a long chain, which is sometimes more convenient. 2 1 CH2CH2CH2OH 3-cyclohexyl-1-propanol 58 Practice methylcyclopentane 1-ethyl-2-methylcyclopentane Structure, Bonding, and Isomerism • Alkenes have the possibility of cis- transisomerism since the pi bond does not permit rotation. • If a molecule has two double bonds between carbon atoms, it is called a “diene.” Double and Triple Bonds 61 Name the following Double and Triple Bonds trans-2-butene Double and Triple Bonds trans-2-pentene Draw a cis, trans-2,4-heptatriene cis,trans,trans,cis-2,4,6,8-decatetraene 65 AROMATIC COMPOUNDS Naphthalene Benzene 66 AROMATIC COMPOUND • See your text for physical properties of these compounds. • Aromatics like benzene have sp2 hybridization with delocalized pi electrons. The delocalized p bonding is the key to these compounds. • They do not undergo addition reactions like alkenes and alkynes, but rather react by way of substitution. 67 Substitution reactions with aromatic compounds, not addition CH3 CH2 CH3 CH2 + Br + Br No Reaction H Br Br C C H H H Br + Br + HBr 68 Naming Aromatic Compounds OH CH3 CH3 CH3 Phenol napthalene Toluene o-Xylene anthracene 69 X ortho -- o meta -- m para -- p CH3 CH3 CH3 CH3 CH3 o-Xylene m-Xylene CH3 p-Xylene 70 Synthesis: substitution or elimination The following are all substitution reaction. Replacing one atom with another. • Alkyl halides (3) • Reaction with aromatic compounds (3) • Making an alcohol (1) • From Alcohols make aldehydes, ketones, and carboxylic acids. The only other type of synthesis is removing an atom, this is called elimination. Preparation of Alkenes and Alkynes • Acetylene aka ethyne, from calcium carbide, CaC2 • Steam cracking for the formation of ethylene, ethene, from ethane. Addition Reactions • Symmetrical addition is simple, but asymmetrical addition follows Markovnikov's rule: the hydrogen adds to the carbon with the most hydrogen. For alkynes, the addition is always two mole to one mole of alkyne, the product being a substituted alkane. If hydrogen gas is added, the process is called hydrogenation. 72 Addition of Hydrogen Halides to Alkenes: Markovnikov's Rule Hydrogen halides (HCl, HBr, HI) add to alkenes: X = Cl, Br, I + H-X A B These reactions may be carried out by adding HX to a solvent such as acetic acid or dichloromethane in the presence of the alkene, or by bubbling gaseous HX into a solution of the alkene. The reactivity order of HX in these additions is: HI > HBr > HCl The reactivity of HCl is very low except with highly substituted alkenes. 73 Regiochemistry of the Addition Reaction Regiochemistry means the specific carbons of the alkene to which the H and X attach. In unsymmetrical alkenes, there are two possible regiochemistries: CH2=CHCH3 + HBr propene Br CH3CHCH3 2-bromopropane Br + HBr 2-methylpropene 2-bromo-2-methylpropane CH3CH2CH2Br 1-bromopropane very little formed CH3CHCH2Br CH3 1-bromo-2-methylpropane very little formed 74 Markovnikov's Rule Vladimir Markovnikov (University of Kazan) in 1869: "The hydrogen of the acid attaches to the carbon that already holds the greater number of hydrogens." This prediction is called "Markovnikov's Rule." CH2=CH-CH3 CH2-CH-CH3 H H Br Br Markovnikov addition product Addition reactions that follow this rule are called Markovnikov Additions. Markovnikov Addition CH3CH2CH2 C CH 1 mol HBr ethanol Br Br 1 mol HBr CH3CH2CH2 C CH3CH2CH2 CH ethanol CH3CH2CH2 Br Br C CH Br Br C CH Br Br The hydrogen adds to the carbon with the most hydrogen ALKENES: Addition 77 Examples of the Halogenation Reaction Chlorination CH3CH2CH=CH2 + Cl2 1-butene CH3CH2CHClCH2Cl - 9o C 1,2-dichlorobutane Bromination + Br2 - 5o C + CCl4/ethanol cyclopentene Br Br Br trans-1,2-dibromocyclope (Racemic Form) ALKENE to ALKANE ALKENES: Elimination Benzene Reactions halogenation Br + Br2 + HBr Benzene Reactions nitration NO2 + HNO3 (conc) H2SO4 (conc) Benzene Reactions alkylation CH2CH2CH2CH3 CH3CH2CH2CH2Cl AlCl3 ALCOHOLS ALCOHOLS Naming Alcohols • The alkane name is modified by dropping the e and adding ol. CH3CH2OH is ethanol • If three OH groups are present, the molecule is called a triol. CH3C(OH)3 is ethantriol Primary, secondary, and tertiary alcohols H C R R R OH H primary or 1° R C OH H secondary or 2° R C OH R tertiary or 3° What type of alcohol’s are these? 1°, 2°, 3°? Metalation of Alcohols Sodium metal reacts with an alcohol to produce hydrogen gas and the sodium alkoxide, refered to as a metalation, since the oxygen is still attached. CH3CH2OH + NaH OH CH3CH2O-Na+ + H2 O-Na+ + NaOH + H2O 89 Chemistry of Alcohols 1. Alcohols can go through substitution, and elimination reactions Which means Alcohols are either oxidized or reduced Formation of ALCOHOLS addition reaction to an alkene H2C CH2 + H2O H3PO4 CH3CH2OH Could also be called an oxidation reaction because we’re adding oxygen to the carbon Substitution • An alcohol reacts with HX to produce the alkylhalide and water, where X is Cl, Br, I • CH3CH2OH + HCl CH3CH2Cl + H2O Elimination • In the presence of concentrated sulfuric acid and heat an alcohol will eliminate water and form an alkene; the reverse of how alcohols are formed. 92 Suggest a method for making Starting with 1 butene make 2-bromo butane Br CH3 CH2 CH CH2 HBr CH3 CH2 ethanol CH CH3 Markovnikov Starting with 1 butene make 1-bromo butane CH3 CH2 CH3 CH2 CH CH2 H3PO4 CH2 CH2 OH CH3 CH2 HBr ethanol CH2 CH2 OH CH3 CH2 CH2 CH2 Br 93 Addition to Ethylene or Elimination by ALCOHOLS H2C CH2 + H2O H3PO4 CH3CH2OH H2SO4 Forward is an oxidation, the reverse a reduction. 94 • Oxidation of Alcohols –Oxidation of Primary Alcohols to Aldehydes » A primary alcohol can be oxidized to an aldehyde or a carboxylic acid • The oxidation is difficult to stop at the aldehyde stage and usually proceeds to the carboxylic acid » A reagent which stops the oxidation at the aldehyde stage is pyridinium chlorochromate (PCC) • PCC is made from chromium trioxide under acidic conditions • It is used in organic solvents such as methylene chloride (CH2Cl2) 95 96 Alcohols can be oxidized to carboxylic acids or ketones 1. primary alcohols to aldehydes with mild Oxidizing agents O PCC CH3CH2 PCC = C OH H3C N+ H CrO3Cl- Pryidinium chlorochromate H Alcohols can be oxidized directly to carboxylic acids or ketones 1. primary alcohols directly to acids by STRONG oxidizing agents O CH3CH2 OH CrO3, H+ C H3C Notice lose of 2H+ OH Alcohols can be oxidized to carboxylic acids or ketones 2. secondary alcohols to ketones O OH PCC CH CH3 CH3 2-propanol C H3C CH3 propanone Notice lose of 2H+ Tertiary alcohols No reaction OH PCC, or CrCl3 CH3 C CH3 no reaction CH3 Why? CARBONYLCOMPOUNDS The carbonyl group is a carbon atom double bonded to an oxygen atom, and is found in aldehydes, ketones, carboxylic acids, and esters. O C 102 NaBH4 or LiAlH4 103 CARBONYL COMPOUNDS CARBONYL COMPOUNDS • The aldehyde has at least one hydrogen atom bonded the carbonyl carbon. • The ketone has two carbon atoms bonded to the carbonyl carbon. • The carboxylic acid has an OH bonded to the carbonyl carbon. • The ester is a combination of an alcohol and a carboxylic acid. • aldehyde, RCOH; carboxylic acid, RCOOH; ketone, RCOR'; ester, RCOOR'. CARBONYL COMPOUNDS • Carboxylic acids can be formed by oxidizing primary alcohols or aldehydes. • Reducing aldehydes and acids with NaBH4 or LiAlH4 produces a primary alcohol. • Reduction of a ketone produces a secondary alcohol. 107 –The Use of Lithium Reagents »Organolithium reagents react similarly to Grignard reagents • Organolithium reagents tend to be more reactive 108 The Use of Sodium Alkynides »Sodium alkynides react with carbonyl compounds such as aldehydes and ketones to form new carbon-carbon bonds 109 Esters • Form from carboxylic acids and alcohols when heated with sulfuric acid. They are named from the alcohol and the acid with an ate ending CH3CH2OH + CH3COOH CH3COOCH2CH3 110 Esters Synthesis of Esters Direct Esterification of Carboxylic Acids Carboxylic acids and alcohols react in the presence of a small amount of strong acid to give esters. COOH COOCH3 + CH3OH Benzoic acid Methanol H+ + H2O Methyl benzoate Esterifications are acid-catalyzed equilibrium reactions. Catalytic amounts of concentrated sulfuric acid or hydrochloric acid are used. Usually a large excess of the alcohol (10- or 15-fold) is used to drive the equilibrium to the product side. Product formation can also be promoted by removing the water as it is formed. 111 This mechanism for esterification is consistent with the incorporation of the isotopic label: H+ O C6H5COCH3 + H2O = = O C6H5COH + CH3OH A Mechanism for Acid-Catalyzed Hydrolysis of Esters Since every step is reversible, the reverse of the esterification scheme is the mechanism for the acid-catalyzed hydrolysis of esters. H+ + H2O O C6H5COH + CH3OH = = O C6H5COCH3 The direction of the reaction is controlled by the relative concentrations of water versus alcohol. 112 Transesterification This is a process whereby the ester of one alcohol may be converted into the ester of a second alcohol by the equilibrium: H+ O RCOR'' + R'OH = = O RCOR' + R''OH An example = O CH2=CHCOCH3 + CH3CH2CH2CH2OH Methyl acrylate H+ Butyl alcohol = O CH2=CHCOCH2CH2CH2CH3 + CH3OH Butyl acrylate Methyl alcohol The equilibrium is shifted to the product side by using an excess of butyl alcohol and/or distilling out the lower boiling methanol from the reaction mixture. Alkyl halides RX • React to form alcohols in water using a strong base like NaOH, substitution reaction. • In an alcohol solvent, the same reactants form an alkene, elimination reaction. CH3 CH2 CH3 CH2 CH2 CH2 Br CH2 CH2 OH NaOH Heat CH3 CH2 CH3 CH2 CH2 CH2 OH CH CH2 Ethers R-O-R’ (Additional material) • Ethers, R-O-R', can be formed from the reaction of alcohols when heated in the presence of concentrated sulfuric acid. • See Lab IVCX 15 2 CH3 CH2 CH2 CH2 OH sulfuric CH3 CH2 CH2 CH2 O CH2 CH2 CH2 CH3 11.6 FATS AND OILS • Fats and oils are esters of glycerol 1, 2, 3-propanetriol • The R group of the triester is a long chain fatty acid. BOOM! FATS AND OILS • Some fats are saturated, some unsaturated, and some are polyunsaturated. • When the triester is hydrolyzed with strong base, the sodium or potassium salt forms and is called a soap. –The process is also called saponfication. Single bonds only Fats Double bonds Fats AMINES AND AMIDES • React as bases and have bad smells • React with carboxylic acids to form amides which are similar in structure to esters. AMINES AMIDES Polymers S U L F U R 11.7 SYNTHETIC POLYMERS • Polymers are formed from combinations of monomers. • They can be classified many ways. –Thermoplastics can be heated and reformed again and again. –Thermosetting plastics are heated and formed, but cannot be heated and reformed because of their high degree of cross-linking. 11.7 SYNTHETIC POLYMERS • Another classification system for polymers is based on their intended use: - plastics - fibers - Elastomers - coatings - adhesives • Polymers can also be classified by the way they form: –addition polymers –condensation polymers Addition Polymers • The monomers for these polymers all have a double bond. • If an appropriate initiator is added, these monomers can add to the chain one at a time by breaking the double bond. • This process is called chain growth polymerization. • Copolymers are formed from a mixture of monomers. 127 Addition: Free Radical Polyethylene: Addition CH2 CH2 CH2 CH2 n Polypropylene CH3 CH CH3 CH2 CH CH2 n (a) Linear, straight Polyethylene Branching Bridging, crosslink Teflon CF2 CF2 CF2 CF2 n What would the following addition polymers look like O C O Cl CH CH2 for PVC CH2 CH3 CH methyl methacrylate for Lucite, Plexiglass CH CH2 stryene for styrofoam PETE (polyethylene terephthalate), HDPE (highdensity polyethylene), LDPE (low-density polyethylene), PP (polypropylene), CLPE (cross-linked polyethylene, V (vinyl) or PVC, also RLDPE (resin mix, already recycled. The # is another way of identifying that polymer. Condensation Polymers • These polymers are usually copolymers. • One monomer is a dicarboxylic acid and the other monomer is either a dialcohol or a diamine. • These polymers are named as polyesters or polyamides. Nylon 66 Polyamide Chains Monomers Polymer 137