* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
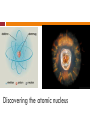
STRUCTURE OF ATOMIC NUCLEUS, RADIOACTIVITY 14. 11. 2013. Physical Basis of Biophysics Nuclear physics Atomic shells investigation - Quantum physics Nuclear physics - This science is not closed. There are more unclarified observations. The structure of the atom Democritos, Dalton, Thomson, Rutherford, Bohr The word atom has the origin: atomos ( uncuttable (indivisible). ) a Greek word meaning ”All matter is composed of atoms, which are bits of matter too small to be seen. These atoms CANNOT be further split into smaller portions.” Democritos Atomic nucleus does not exist! Atomic models based on experiments: : Thomsons’ atomic model(1906) + _ electrons • Discovery of the electron. • The plum-pudding model. • There is no nucleus mentioned. The positive charge is spread in a ”large” volume. + + + + _ Rutherford model (1911) Discovery of nucleus. Positively charged nucleus exists and the electrons move in the substance. Neutron and proton are not mentioned! The positive -particles are diverted because of the positive charge of the nucleus. (repulsion) Bohr model (hydrogen atom; 1913) The electron travels around the nucleus on a circular orbit. The parameters of orbits are quantized: energy, angular momentum, radius Neagtively charged electrons→ in electron clouds (maximum distance~10-10 m) Positively charged nucleus→ proton and neutron were not known! The nucleus is uncuttable. What does an atom consist of? According to Bohr-Sommerfeld’s model (1915): Negatively charged electrons → e- clouds Positively charged nucleus → proton and neutron were not known! Spatial connection between the nucleus and electron: it can not appear everywhere in the substance, just close to the nucleus! (maximum ~10-10 m) Discovering the atomic nucleus The stucture of the nucleus proton neutron nukleon It determines the chemical element, the proton number. The volume of the nucleus is 100 000 –fold smaller than the whole volume of the atom. Discovery of proton Ernest Rutherford in1918 During investigation of the nitrogen gas he observed that the hydrogen can be detected when alfa-particles hit into the nitrogen gas. He detected, that the hydrogen can come just from the nitrogen. Nitrogen have to contain the hydrogen. The word proton has the origin: protos a Greek word meaning: first (primary) Exploring the neutron Rutherford’s nucleus alteration experiment (1917) Irradiated nitrogen gas with α-particles (He2+): 14 7 N 4 2 He 17 8 O 11H Internuclear reactions took place! Alteration of chemical elements occurs not only by radioactive decomposition. Ernest Rutherford 1871-1937 Experiment of Bothe and Becker (1930.) They bombarded Beryllium with α-particles, and detected a ray with high penetration ability, which did not diverge in magnetic or in electric field. → Neutral! Walther Bothe 1891-1957 (Nobel-prize,1954) Chadwick’s interpretation (1932) Collision of Be and α-particle → new particle is emitted. Same mass as a proton but without any electric charge. 9 4 4 2 Be He 12 6 C 1 0 n He named this new particle: neutron. neutros: Greak word, neutral James Chadwick 1891-1974. (physical Nobel-prize, 1935.) Heisenberg and Tamm (1932) They developed a new nuclear model which includes neutrons, as well. New meaning is brought to atomic number! 12 6 C Mass number (A) Proton number (Z) or atomic nuber (charge) N = A-Z; neutron number Properties of Particles Name Mass (kg) Electrical charge(C) Proton 1,673∙10-27 1,6∙10-19 Electron 9,109∙10-31 - 1,6∙10-19 Neutron 1,675∙10-27 0 Symbol Relative mass Relative charge p+ 1 +1 e- 1/1840 -1 n0 1 0 Types of nuclei According to construction isotope: same proton number, different neutron number (11H and 21H) nuclide: same proton and neutron number A Z A Z X A 4 Z 2 X X 4 2 He 2 2e Relative atomic mass It is a dimensionless physical quantity, the ratio of the average mass of atoms of an element (from a single given sample or source) to 1/12 of the mass of an atom of carbon-12. The amount of substance: The SI unit for amount of substance is the mole. The mole is defined as the amount of substance that contains an equal number of elementary entities as there are atoms in 12 g of the isotope carbon-12. This number is called Avogadro's number and has the value 6*1023 Molar mass: M=m/n (g/mol) Problems 1. How many protons and electrons are in 10847 Ag-atom? 47 proton and 47 elektron 2. How many protons and neutrons are in 35 g 73Li-atom? 3. How many protons and neutrons are in the Ca2+ ion? From the simplest: Hydrogen atom „atom engineering” p+ Size? RHatom ≈ 10 -10 m; 1 proton, nothing more! 1 1 H RHnucleus ≈ 10 -15 m More complex atom like: He (atomic number = 2) + + The charged protons repel each other because of the electrostatic Coulomb force. The real He atom 0 + + Atomic number = 2, mass number = 4 0 2 p+ and 2 n0 4 2 He The presence of neutrons does not explain the stability (electrically neutral)! But they demonstrably stabilize the nucleus. Some ”glueing effect” should appear! Stronger than the electric repulsion! Neutrons also take part in creating this force which is not based on electric charge! What is that force? What are interaction in the nucleus? Nuclear force – strong interaction Binding energy Compensates the repulsion of electric charges. high intensity (strong) short range of interaction (10-15 m) attraction only (always) independent of electric charge neutrons are also included! in p-p, p-n, n-n interactions the same magnitude of force is created Deficit of mass → binding energy The mass of composite nuclei is always less than the total mass of its components (separated protons and neutrons). The missing mass value is linearly proportional to the binding energy. Energy is disengaged (released), while a nucleus is constructed from free nucleons. m ( Z m pr E m c 2 N mn ) mnucleus Mass-energy equivalency rule of Einstein (Nuclear) Binding energy: is the required energy to remove one nucleon from the nucleus of an atom. (c: speed of light) Interactions Nuclear models Increase of nucleon number - effects + 0 0 Increasing: nucleon number → mass (number: A) + 1 • radius of atom r ~ A 3; A • volume of atom 3 N Z V ~r ~ A • surface of atom 2 surface ~ r ~ A 2 EB Same effect(s) as in the case of liquid drops! nonlinear! V~A Read this out like: volume is linearly proportional to the mass number 0 A 3 Liquid drop model (LDM) incompressible nuclear fluid Experimental observations: 1. Each of the nucleons is bound with (almost) the same binding energy. (EBneutron = EBproton !) 2. Total binding energy of the nucleus is proportional to the number of nucleons (A). 3. The volume of the nucleus is linearly proportional to the number of nucleons (A). Liquid drop model (LDM) 4. size-independent density → incompressibility, 5. spherical form, 6. nucleons interact only with their closest neighbours. Based on macroscopic properties (experimental data). Explains: binding energy, mass, stability of nuclei. Model (1935): created by Carl von Weizsäcker based on the calculations of Hans Bethe. EB A A 2 3 Z A EB Evolume Esurface 2 1 3 A 2Z A ECoulomb EPauli 2 A Eanti 2 3 Hund Binding energy per Nucleon (MeV) One nucleons’ binding energy as a function of atomic number The ratio of surface and volume (energies) changes! (r2/r3 = 1/r) Effect of Coulomb force increases! Atomic number (atomic mass unit) Maximum: between 55-60! The fit is almost good! But...! The model predicts: 62! What’s wrong with the liquid drop model? 27 Binding energy per Nucleon (MeV) magic numbers: N or Z = 2, 8, 20, 28, 50, 82, 126 These nuclei are more stable than the LDM predicts. Atomic number (atomic mass unit) The phenomenon is similar to the magic numbers of the electron shells: 2, 8,18, 32 Halogens have more stable electron structure because of closed e--shells! Reason: These atoms contain closed (filled) atomic shells. That phenomenon is not taken into account in the LDM! What can we do? Is there any better model? Atomic shell model (sphere symmetric) Bartlet, Elsasser, 1934: „independent particle model” Jensen and Göppert-Mayer, 1949: atomic shell model • The nucleons Schrödinger equation’s with quantized parameters (energy, angular momentum, magnetic momentum, spin) → characterise the atomic shells. (spin value: ½, hence Pauli’s-principle is valid) • Atoms with closed atomic shells are more stable! protons and neutrons separately fill their own energy levels ASM Ep En 0 eV 29 1 1 H hydrogen 2 1 H deuterium 3 1 H tritium 4 2 lowest energy level He helium This theory explains the first three (2,8,20) magic numbers! But several experimental results are not confirmed! The „Unified nuclear model” explains properly in details every nuclear property. This model is not required to know! Radioactivity Is the radioactivity a natural process? Yes What provokes the transmutation? The instability of the nucleus! What kind of utilization do you know? Nuclear power plant, atom bomb, diagnostics, therapy Isotopes There are various form of nucleus (of chemical elements). Isotopes include the same number of protons but different numbers of neutrons. Hydrogen: 1 p+ + different numbers of neutron - 1: 1H (1 p+) Hydrogen - 2: 2H (1 p+ + 1 n0) Hydrogen - 3: 3H (1 p+ + 2 n0) Hydrogen hydrogen deuterium tritium The stabiliy of nucleus is defined by the attractive effect between nukleon and repulsive effect between protons. In case of the stabil isotopes (low atomic number) the number of protons and neutrons differ slightly. Unstable nucleus: The nuclei try to reach more stable state with lower energy They can emit photons. Particles are emitted/radiated Radioactivity The radiactive radiation is the results of nucleus transmutation. There are about ~ 40 nature form of radiactive isotopes. Discovery of radioactivity Becquerel: He observed that the uranium caused black spot on non-illuminated photosensitive plate. What Becquerel had discovered was that a piece of mineral which contained uranium could produce it's image on a photographic plate in the absence of light. Pierre Curie (1859-1906) and Marie Curie (1867-1934) They compared the activity of pure uranium to a uranium ore sample, they found that the ore was significantly more radioactive than the pure material. They concluded that the ore contained additional radioactive components besides the uranium. This observation led to the discovery of two new radioactive elements which they named polonium and radium. Binding energy per nucleon as a function of mass number Stability of the nucleus 56 26 Fe Ways to reach Stability Fission Nuclear reactors, atomic bomb Fusion stars http://outreach.atnf.csiro.au/education/senior/cosmicengine/sun_nuclear.html http://www.princeton.edu/~chm333/2002/spring/Fusion/tour1/index.htm Radioactive radiations -decay » -radiation A Z A Z X X A 4 Z 2 X 4 2 He A 4 Z 2 X 226 88 Ra Maximal exit speed: 15 000 000 m/s (0,05 c) Line spectrum (characteristic) 238 92 226 222 210 U , 241 Am , Ra , Rn , 95 88 86 84 Po 222 86 Rn 4 2 -decay » -radiation Negative -decay Experiment: Curie 1911 n 0 A Z X p A Z 1 X e νe e e electron 137 55 Cs νe 137 56 Ba e Maximal exit speed: 180 000 000 m/s (0,6 c) Continuous spectrum (antineutrino) Isotopes: 3 1 14 6 137 55 132 53 40 19 H , C , Cs, I , K -decay » -radiation Positive -decay p A Z X n0 e A Z 1 X e νe positron e 122 11 Na νe 22 10 Ne e isotopes 11 6 22 11 C , Na -radiation Side effect of - or -radiation! Electromagnetic radiation ( -photon) f>1019 Hz, and E>100 keV Created at energy transitions of nucleus, from excited state to ground state. Propagates at light speed Line spectrum (characteristic) 22 11 40 132 Na,19 K ,137 Cs , 55 53 I -photon 137m 56 Ba 137 56 Ba m: metastable state Radiations – base of comparison External influence not required Physical and chemical environment has no influence Ionisation (physics) Chemical and biological effects Physical properties: Activity Lifetime Spectrum Penetration LET (linear energy transfer) Comparison Mean lifetime 222 86 226 88 210 84 238 92 Rn, Ra, Po, U 4 s; 11 days; 138 days; 4,5∙109 years - 132 3 14 40 53 1 6 19 8 days; 12 years; 5568 years; 1,2∙109 years; 7,6∙10-22 s + 11 6 I , H , C, K 22 11 C , Na 20 m; 15 h 22 40 137 132 11 19 55 53 2,6 years; 1,2∙109 years; 26 years; 8days Na, K , Cs, I Comparison LET (ions/mm) Penetration depth, range Linear (characteristic) high 8-10 000 Law Air: cm Plexi-glass: mm Continuous (because of anti/neutrino) average 6-8 Linear (characteristic) law 0,1-1 Spectrum Average Air: m Plexi-glass: cm Lead : mm High Lead: cm Activity(A) Radioactive transmutation is a random process! Number of transmutating nuclei in 1 sec. transmutation = decay Unit: Becquerel 1 Bq = 1 transmutation /sec. Attention! Radioactive ”decomposition” does not mean that the atom disappears! Do not use ”disintegration” for the process Old unit: Curie (1 Ci = 3.7 ∙ 1010 Bq) Stable isotope Radioactive isotpe Daughter atom Decay law N(0) : number of radioactive nuclei at the begining N(t) : number of remaining radioactive nuclei at any moment ”t” Decay constant ( ): Characterises the rate of decomposition. Defines the probability of transmutation of one nucleus. A Number of remaining radioactive nulei N(0) T1 N (t ) N ( 0) 2 2 N(0)/2 N(0)/e T1/2 N (t ) Mean lifetime( ): Reciprocal of the decay constant. t 1 time t N (t ) N ( 0) e Half life– mean lifetime The time required for ½ the amount of a radioactive material to disintegrate. Phosphorus-32 radioactively decays to form Sulfur-32 Half life 32P = 14 days t N (t ) N ( 0) 2 T1 t N (t ) N ( 0) e t 2 2 T1 t 2 1 ln 2 T12 e 1,443 T12 Problems Half life 1. The intensity of the radiactive sample is 8300 cpm. After15 days the intensity is 6457 cpm. Calculate the half-life! 41,4 days Activity 2. When you make an experiment with 86Rb, the Geiger Müller-detector shows 21000 cpm count rate at a distance of 2 cm from the test tube. What would be the distance from the tube, when the detector shows 840 cpm? The number of detected particles is inversely proportional to the square of the distance. N(far)= N(near)/(r2/r1)2 10 cm Homework 1. How many protons and neutrons are in 5 g 40 19 K –atom? s s 2. How many is the decay constant of the radioactive sample is the half-life is 3 years. 1 1,443 T12 1 ln 2 T12 λ=0.23 3. Determine the activity of unknown radioactive Cd isotope if the activity is 1540 Bq after 21 days and the half-life is 4 days! Thank you for your attention!